Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Matúš | -- | 1998 | 2023-07-04 18:40:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 1998 | 2023-07-10 03:27:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matúš, P.; Littera, P.; Farkas, B.; Urík, M. Aspergillus and Penicillium Species in Biodegradation of Pesticides. Encyclopedia. Available online: https://encyclopedia.pub/entry/46411 (accessed on 07 February 2026).
Matúš P, Littera P, Farkas B, Urík M. Aspergillus and Penicillium Species in Biodegradation of Pesticides. Encyclopedia. Available at: https://encyclopedia.pub/entry/46411. Accessed February 07, 2026.
Matúš, Peter, Pavol Littera, Bence Farkas, Martin Urík. " Aspergillus and Penicillium Species in Biodegradation of Pesticides" Encyclopedia, https://encyclopedia.pub/entry/46411 (accessed February 07, 2026).
Matúš, P., Littera, P., Farkas, B., & Urík, M. (2023, July 04). Aspergillus and Penicillium Species in Biodegradation of Pesticides. In Encyclopedia. https://encyclopedia.pub/entry/46411
Matúš, Peter, et al. " Aspergillus and Penicillium Species in Biodegradation of Pesticides." Encyclopedia. Web. 04 July, 2023.
Copy Citation
Since filamentous fungi of Penicillium and Aspergillus genera can colonize very diverse niches, and Ascomycota seems to be the dominant phylum within the microbial group in various contaminated substrates, they possess great potential in the remediation of pesticide-contaminated sites. Different species can remove the pesticides at different rates, and to various extents; however, the fungal ability to resist high concentrations of pesticides is almost unparalleled compared to other microbial groups. Their performance may be further improved by applying indigenous strains isolated from pesticide-contaminated soils and sediments.
biotransformation
filamentous fungi
organochlorine
organophosphorus pesticides
1. Introduction
The current agricultural practice is contingent on the use of various pesticides since there is an urgent need to enhance the crop production to supply the rapidly increasing food-demand [1]. Throughout the globe, approximately 2 million tons of pesticides are produced and utilized annually, and it was estimated that in 2020, the global pesticide usage was approximated to be 3.5 million tons [2]. The application of pesticides is apparently advantageous since it diminishes the crop infestations, and thus, limits the harvest losses and positively affects the crops quality [3]. However, due to their potent biological activity as toxins and owing to their extensive or injudicious application, the heavy soil treatment with pesticides can endanger the wildlife. The pesticides and their toxic degradation products can enter the plant tissues and build up in the food chain or remain in the soil and water environments and negatively affect the soil fertility and water quality [4]. Thus, the pesticides pose a significant risk to the environment and to human health. The alarming increase in the production and usage of pesticides triggered the notion to reduce the impact of pesticides and find sustainable alternative solutions to protect the crops [5].
Although the role of many pesticides in the environmental deterioration has not been adequately resolved, it is indisputable that they have adverse effects on various non-target organisms [6]. Thus, the research on advanced practices protecting wildlife, which highlights a more cautious use of synthetic agrochemicals, careful risk assessment, and licensing, is very much needed [7]. Therefore, the development of ecofriendly technologies focused on reducing the utilization of synthetic pesticides, especially those with high persistence in the environment, has been addressed [8][9]. More importantly, there is still an issue of developing a proper treatment technology for the remediation of pesticide-contaminated soils. This is a complex problem, since the areas with point-source contamination are usually agricultural soils whose properties should be maintained; thus, aggressive technologies should be omitted [10]. Among more recent and ecologically acceptable emerging technologies is bioremediation, which involves the utilization of indigenous microflora, adapted or genetically engineered microorganisms or their enzymes for the degradation and conversion of pesticides to another form via co-metabolism or mineralization [11][12].
2. Degradation of Organochlorine Pesticides by Aspergillus and Penicillium Species
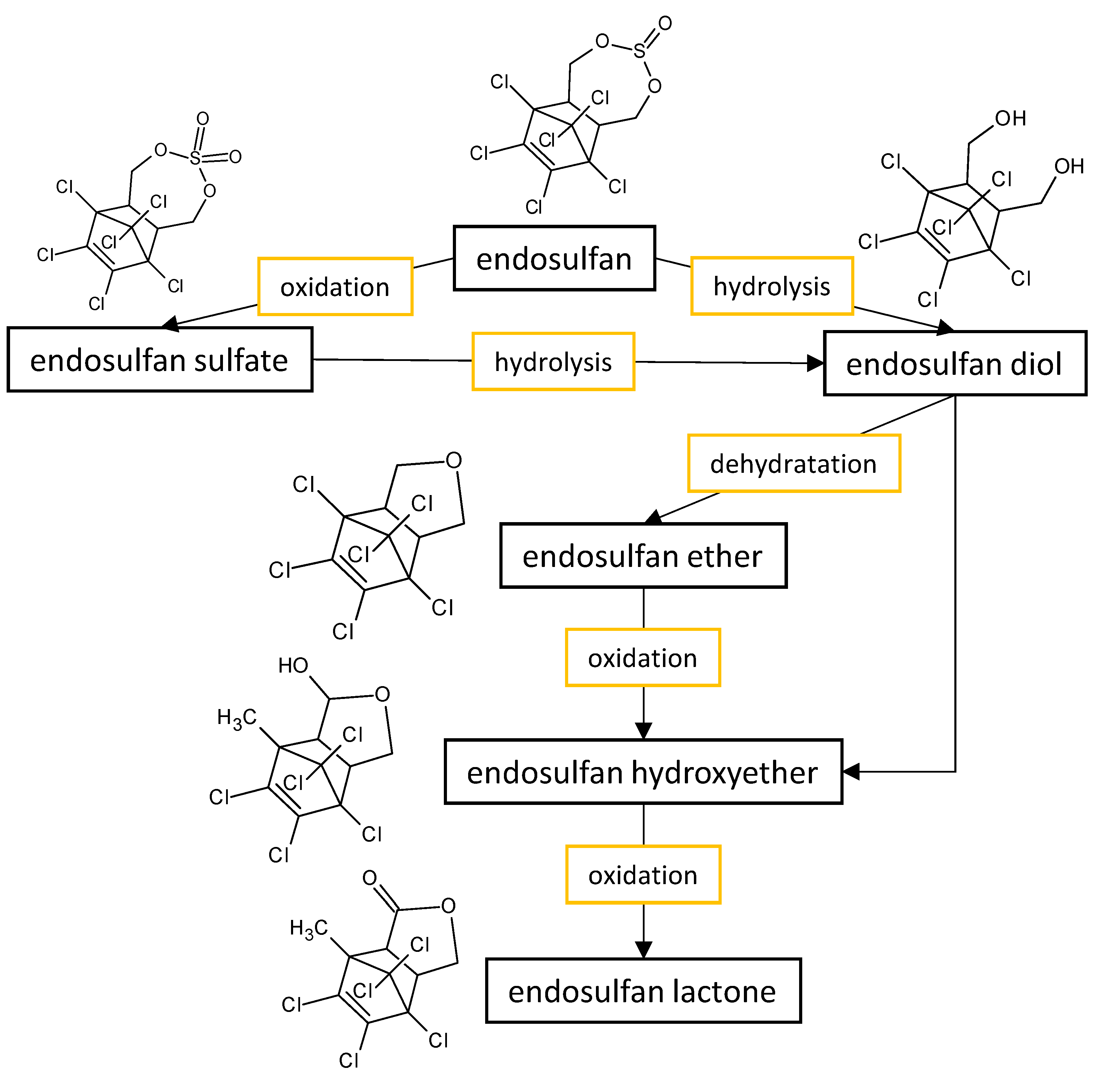
The fungal performance in the biodegradation of the most common organochlorine pesticides (endosulfan and lindane) in culture media, as well as the transformants or end products (Figure 1) formed by the fungi belonging to Aspergillus and Penicillium genera are listed in Table 1.

Figure 1. Proposed metabolic pathway for the degradation of endosulfan by Aspergillus and Penicillium fungal strains.
Table 1. Cultivation conditions and reported performances of filamentous fungi belonging to the genera Aspergillus and Penicillium in the biodegradation of organochlorine pesticides.
| Fungal Strain | Degradation Efficiency | Cultivation Conditions | Degradation Products | Reference |
|---|---|---|---|---|
| Penicillium sp. CHE23 | 94.8% | modified mineral salt medium with ~57 mg·L−1 of endosulfan as carbon source incubated for 144 h at 30 °C and 150 rpm | not reported | Romero-Aguilar et al. [13] |
| Aspergillus niger | complete degradation | Czapek–Dox broth with 350 mg·L−1 of β-endosulfan source incubated for 120 h at 30 °C and 120 rpm | endosulfan sulfate being most persistent metabolite; after 120 h, complete mineralization was suggested | Bhalerao and Puranik [14] |
| Aspergillus niger ARIFCC 1053 | complete degradation | Czapek–Dox broth with 1000 mg·L−1 of technical-grade endosulfan incubated for 7 days at 30 °C and 120 rpm | complete mineralization | Tejomyee [15] |
| Aspergillus niger | 98.6% | Czapek–Dox broth with 15.4 µg·g−1 of technical-grade endosulfan incubated for 15 days at 30 °C and intermittent shaking | not reported | Mukherjee and Gopal [16] |
| Aspergillus terreus, (Cladosporium oxysporum) |
91.5%, (89%) |
potato dextrose broth with 1.89 µg·g−1 of technical-grade endosulfan incubated for 15 days at 25 °C | trace concentrations of endosulfan sulfate were detected during incubation; no end products were reported | Mukherjee and Mittal [17] |
| Aspergillus terricola, Aspergillus terreus, (Chaetosartorya stromatoides) |
~90% | non-sulfur medium enriched with 100 mg·L−1 of α or β endosulfan isomers incubated for 12 days at 30 °C and 150 rpm (pH 6) | major metabolic products being endosulfan diol and endosulfan ether | Hussain et al. [18] |
| Aspergillus niger AE | complete degradation (0.1% endosulfan), and 76% (0.5% endosulfan) | Czapek–Dox broth spiked up to 5000 mg·L−1 (0.5%) of endosulfan incubated for 8 days at 30 °C and 180 rpm (optimum at pH 4) | not reported | Mukhtar et al. [19] |
| Aspergillus niger, Aspergillus flavus, Penicillium chrysogenum |
77% (AN), 72% (AF), 69% (PC) |
potato dextrose broth with 10 mg·L−1 of endosulfan incubated for 35 days at 29 °C | desulphurized transformants of endosulfan, while chlorine atoms remained imperforated | Ahmad [20] |
| Aspergillus sydoni | 95% (α isomer), 97% (β isomer) |
Czapek–Dox broth with 100 mg·L−1 of α or β endosulfan isomers incubated for 18 days at 30 °C and 150 rpm | major metabolic products being endosulfan sulfate | Goswami et al. [21] |
| Aspergillus tamarii JAS9, (Botryosphaerialaricina JAS6) |
kinetic analysis shows that 50% of α and β endosulfan isomers were degraded in 1.7 and 2.2 days by JAS9, respectively (4.2 days for 50% reduction of β endosulfan by JAS6) | M1 medium with 1000 mg·L−1 of technical-grade endosulfan as carbon source incubated for 10 days at 30 °C and 120 rpm | not reported | Silambarasan and Abraham [22] |
| fungal consortium (Botryosphaeria laricina JAS6, Aspergillus tamarii JAS9 and Lasiodiplodia sp. JAS12) | complete degradation (a 50% degradation calculated on the 3rd day of incubation) | M1 medium with 1000 mg·L−1 of technical-grade endosulfan as carbon source incubated for 120 h at 30 °C and 120 rpm | complete mineralization | Abraham and Silambarasan [23] |
| Penicillium camemberti | 70% | acetate-free basal medium supplemented with 1 mM lindane incubated for 120 h at 25 °C and 80 rpm (pH 5) | not reported | Taşeli [24] |
| Aspergillus fumigatus | complete degradation | lindane initial concentration is not indicated; medium (Sabouraud dextrose broth or Nutrient broth) incubated for 5 days at 25 °C | not reported | Kumaravel et al. [25] |
| Penicillium miczynskii CBMAI 93 | 90% | culture medium of artificial salt water supplemented with 50 mg·L−1 of dieldrin incubated for 14 days at 32 °C and 130 rpm | no intermediate degradation products were detected, suggesting dieldrin mineralization of conjugation | Birolli et al. [26] |
3. Degradation of Organophosphorus Pesticides by Aspergillus and Penicillium Species
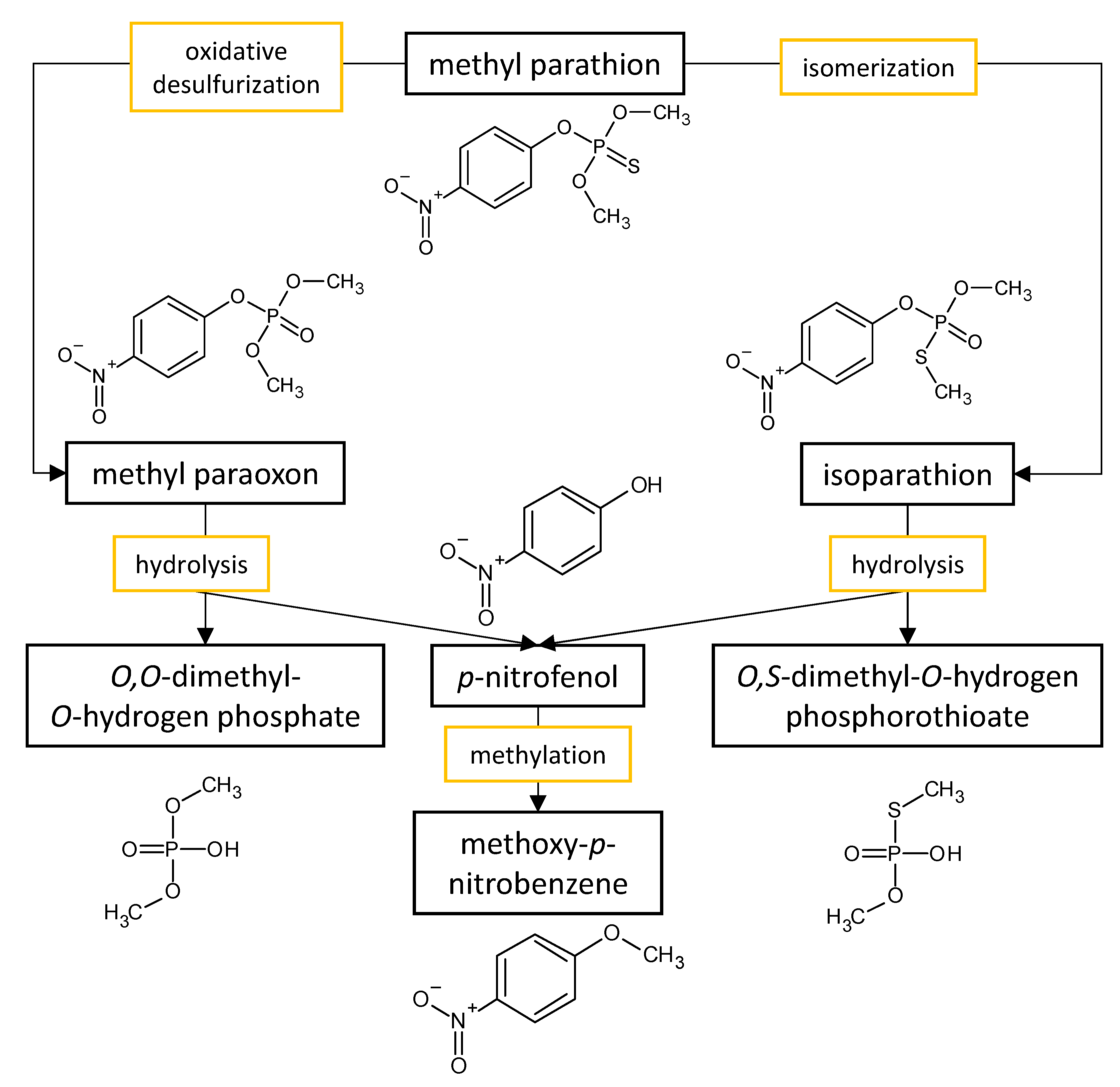
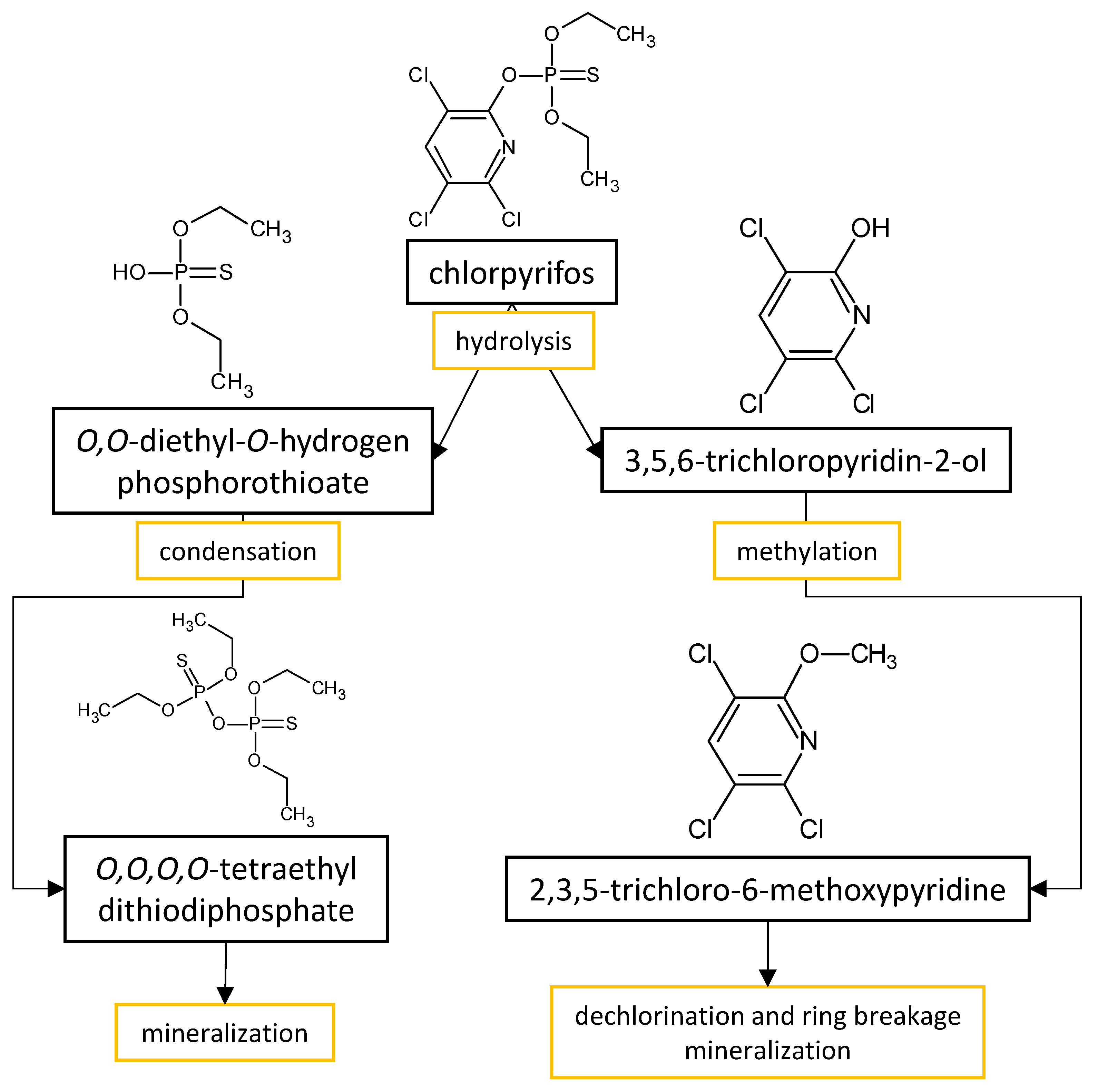
The performances of strains belonging to Aspergillus and Penicillium genera in the biodegradation of organophosphorus pesticides in culture media are listed in Table 2. The summarized biodegradation pathways for methyl parathion and chlorpyrifos, the most studied organophosphorus pesticides, are depicted in Figure 2 and Figure 3, respectively.

Figure 2. Proposed metabolic pathway for the degradation of methyl parathion by Aspergillus and Penicillium fungal strains.

Figure 3. Proposed metabolic pathway for the degradation of chlorpyrifos by Aspergillus and Penicillium fungal strains.
Table 2. Cultivation conditions and reported performances of filamentous fungi belonging to the genera Aspergillus and Penicillium in the biodegradation of organophosphorus pesticides.
| Fungal Strain | Degradation Efficiency | Cultivation Conditions | Degradation Products | Reference |
|---|---|---|---|---|
| Aspergillus sydowii CBMAI 935 | 32%, 80% and 52% of chlorpyrifos, methyl parathion, and profenofos, respectively | 2% malt liquid medium with 50 mg·L−1 of chlorpyrifos, methyl parathion or profenofos incubated for 30 days at 32 °C and 130 rpm | chlorpyrifos degradation resulted in tetraethyl dithiodi- phosphate and 2,3,5-trichloro-6-methoxypyridine; methyl parathion hydrloyzed into methylated phosphate and phosphorothioates, and 1-methoxy-4-nitrobenzen; profenofos degraded into 4-bromo-2-chloro-1-methoxybenzen and O,O-diethyl-S-proyl phosphorothioates |
Soares et al. [27] |
| Aspergillus sydowii CBMAI 935, Penicillium decaturense CBMAI 1234 |
80% | liquid mineral medium supplemented with KNO3 and 100 mg·L−1 of methyl parathion incubated for 30 days at 32 °C and 130 rpm | p-nitrophenol | Alvarenga et al. [28] |
| Aspergillus niger AN400 | 2% (glucose-free treatment, initial concentration of methyl parathion was 19.1 mg·L−1), 43% (glucose-treated medium, initial concentration of methyl parathion 24.9 mg·L−1) |
glucose-free or glucose treated distilled water supplemented with Vishniac solution and up to 24.9 mg·L−1 of methyl parathion incubated for 27 days at 30 °C and 200 rpm | not reported | Marinho et al. [29] |
| Penicillium citrinum, (Fusarium proliferatum) |
complete degradation (the biotic control had the same degradation efficiency) | 3% malt liquid medium with 30 mg·L−1 of methyl parathion incubated for 30 days at 32 °C and 130 rpm | not reported | Rodrigues et al. [30] |
| Aspergillus niger MRU01 | 70%, 54%, 58%, and 68% of malathion, parathion, chlorpyrifos and dimethoate, respectively | Czapek–Dox broth spiked with 500, 470, 260 and 680 μmol·L−1 (0.5%) of malathion, parathion, chlorpyrifos, and dimethoate, respectively, incubated for 5 days at 26 °C and 120 rpm (optimum at pH 4) | not reported | Mohapatra et al. [31] |
| Aspergillus flavus | complete degradation | mineral salt medium supplemented with 5 mg·L−1 of malathion incubated for 36 days at 30 °C (pH 7) on a rotatory shaker (optimized conditions) | not reported | Derbalah et al. [32] |
| Aspergillus sp. F1 | over 90% (89% at an inlet load of 180 mg·L−1·d−1) |
bioreactor supplemented with 300 mg·L−1 of chlorpyrifos as the sole carbon source incubated at 28 °C (pH 7) with dissolved oxygen concentration of 5.8 mg·L−1 (optimized conditions) | not reported | Yadav et al. [33] |
| Aspergillus fumigatus | 99% | potato dextrose broth supplemented with chlorpyrifos (10%) incubated for 9 days at 25 °C (pH 7) and 180 rpm | not reported | Anggreini et al. [34] |
| Aspergillus fumigatus | 95.9% | potato dextrose broth with chlorpyrifos (1.5%) incubated for 5 days at 25 °C (pH 7) and 180 rpm | not reported | Anggreini et al. [35] |
| Aspergillus terreus JAS1 | complete degradation (after 24 h) | M1 medium supplemented with 300 mg·L−1 of chlorpyrifos as the sole carbon source incubated for 96 h at 30 °C and 120 rpm | 3,5,6-trichloropyridin-2-ol that was completely degraded after 48 h; no other metabolites were reported | Silambarasan and Abraham [36] |
| Aspergillus oryzae strains AM1 and AM2 | 73% (AM1), 50% (AM2) |
Czapek–Dox broth spiked with 20 mg·L−1 of chlorpyrifos incubated for 30 days at 25 °C and 60 rpm (optimum at pH 4) | not reported | Barberis et al. [37] |
| Aspergillus viridinutans, Penicillium implicatum | 44.6% (A. viridinutans), 16.2% (P. implicatum) |
potato dextrose broth with 20 mg·L−1 of chlorpyrifos incubated for 14 days at 28 °C | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Abdel-Wareth and Abd El-Hamid [38] |
| Aspergillus niger, (Trichoderma viride) |
72.3%, (95.7%) |
Czapek–Dox broth spiked with 1.25 mg·L−1 of chlorpyrifos incubated for 14 days at 30 °C and intermittent shaking (pH 6.8) | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Mukherjee and Gopal [39] |
| Penicillium citrinum, Aspergillus niger, Aspergillus oryzae |
25.9% (P. citrinum), 64% (A. niger), 50.8% (A. oryzae) | Burkes mineral broth with 10 mg·L−1 of chlorpyrifos incubated for 15 days at 27 °C without shaking (pH 7.2) | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Abd-Alrahman and Mostafa [40] |
| Aspergillus niger | ~80% | soil extract medium with 10 mg·L−1 of chlorpyrifos incubated for 30 days at 25 °C and 60 rpm | 3,5,6-trichloro-2-pyridinol were detected below the concentration of 1 mg·L−1 | Karas et al. [41] |
| Aspergillus fumigatus, A. flavus, A. niger, A. ochraceus, A. tamarii, A. terreus, Penicillium chrysogenum, P. brevicompactum, P. citrinum, P. funiculosum | phosphor mineralization efficiencies ranged from 4 to 46% (Cyolan), from 9.5 to 26.8% (Malathion), and from 2.3 to 6.7% (Dursban) | Czapek–Dox broth spiked with 100 mg·L−1 of cyolan, malathion, and chlorpyrifos incubated for 35 days at 28 °C without shaking | not reported; media and biomass were analyzed for phosphor and sulfur that mineralized from the degradation of insecticide |
Omar [42] |
| Penicillium oxalicum ZHJ6 | complete degradation | mineral salt medium with 1% glucose supplemented with 1 mg·L−1 of methamidophos as sole nitrogen source incubated for 12 days at 25 °C and pH of 5.0 (most favorable conditions) | inorganic phosphor, CH3SH, and CH3OH are hypothesized being formed | Zhao et al. [43] |
| Aspergillus oryzae A-F02 | ~13.7% | fermentation medium with 1.5 g·L−1 of glyphosate incubated for 144 h at 30 °C and 150 rpm | aminomethylphosphonic acid and methylamine, the latter being further degraded | Fu et al. [44] |
4. Biotransformation of Pesticide Belonging to Some Other Chemical Groups by Aspergillus and Penicillium Species
The biodegradation of three pesticides of the different chemical classes, difenoconazole, pendimethalin, and terbuthylazine, by Penicillium brevicompactum and A. oryzae was studied by Pinto et al. [45]. After an 8-day incubation, 99% of pendimethalin was degraded by both fungal strains. Fungi A. oryzae and P. brevicompactum were capable of degrading 88% and 92.7% of difenoconazole, while lower removal percentages were exhibited with terbuthylazine approximating 78% and 71%, respectively. More importantly, the authors have highlighted adsorption as a potential mechanism of pesticide removal, characterized by the fast initial removal rates, which may be an efficient mechanism utilized by the fungus to block the xenobiotic uptake.
Derbalah et al. [46] reported that approximately 93% of the initial famoxadone concentration was degraded within four weeks by strains of A. niger EB2 and Penicillium sp. EB3 and the negligible spontaneous degradation was observed in control experiments. The bioassay conducted with Alternaria solani using the spent medium with degradation products collected at the end of famoxadone treatment showed only slight antifungal activity, indicated by 5% growth inhibition of A. solani.
References
- Pujar, N.K.; Premakshi, H.G.; Ganeshkar, M.P.; Kamanavalli, C.M. Biodegradation of Pesticides Used in Agriculture by Soil Microorganisms. In Enzymes for Pollutant Degradation; Mulla, S.I., Bharagava, R.N., Eds.; Springer Nature: Singapore, 2022; pp. 213–235.
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446.
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Cleaner Prod. 2021, 283, 124657.
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138.
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835.
- Köhler, H.-R.; Triebskorn, R. Wildlife Ecotoxicology of Pesticides: Can We Track Effects to the Population Level and Beyond? Science 2013, 341, 759.
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60.
- Sehrawat, A.; Phour, M.; Kumar, R.; Sindhu, S.S. Bioremediation of Pesticides: An Eco-Friendly Approach for Environment Sustainability. In Microbial Rejuvenation of Polluted Environment: Volume 1; Panpatte, D.G., Jhala, Y.K., Eds.; Springer: Singapore, 2021; pp. 23–84.
- Chatterjee, A.; Mandal, M.K.; Chaurasia, N. Microbial services in agro-environmental management. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbes in Soil, Crop and Environmental Sustainability; Singh, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–272.
- Morillo, E.; Villaverde, J. Advanced technologies for the remediation of pesticide-contaminated soils. Sci. Total Environ. 2017, 586, 576–597.
- Dash, D.M.; Osborne, W.J. A systematic review on the implementation of advanced and evolutionary biotechnological tools for efficient bioremediation of organophosphorus pesticides. Chemosphere 2023, 313, 137506.
- Bhatt, P.; Bhatt, K.; Chen, W.-J.; Huang, Y.; Xiao, Y.; Wu, S.; Lei, Q.; Zhong, J.; Zhu, X.; Chen, S. Bioremediation potential of laccase for catalysis of glyphosate, isoproturon, lignin, and parathion: Molecular docking, dynamics, and simulation. J. Hazard. Mater. 2023, 443, 130319.
- Romero-Aguilar, M.; Tovar-Sánchez, E.; Sánchez-Salinas, E.; Mussali-Galante, P.; Sánchez-Meza, J.C.; Castrejón-Godínez, M.L.; Dantán-González, E.; Trujillo-Vera, M.Á.; Ortiz-Hernández, M.L. Penicillium sp. as an organism that degrades endosulfan and reduces its genotoxic effects. Springerplus 2014, 3, 536.
- Bhalerao, T.S.; Puranik, P.R. Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger. Int. Biodeterior. Biodegrad. 2007, 59, 315–321.
- Tejomyee, S.B. Biominerlization and Possible Endosulfan Degradation Pathway Adapted by Aspergillus niger. J. Microbiol. Biotechnol. 2013, 23, 1610–1616.
- Mukherjee, I.; Gopal, M. Degradation of beta-endosulfan by Aspergillus niger. Toxicol. Environ. Chem. 1994, 46, 217–221.
- Mukherjee, I.; Mittal, A. Bioremediation of Endosulfan Using Aspergillus terreus and Cladosporium oxysporum. Bull. Environ. Contam. Toxicol. 2005, 75, 1034–1040.
- Hussain, S.; Arshad, M.; Saleem, M.; Zahir, Z.A. Screening of soil fungi for in vitro degradation of endosulfan. World J. Microbiol. Biotechnol. 2007, 23, 939–945.
- Mukhtar, H.; Khizer, I.; Nawaz, A.; Asad Ur, R.; Ikram Ul, H. Biodegradation of endosulfan by Aspergillus niger isolated from cotton fields of Punjab, Pakistan. Pak. J. Bot. 2015, 47, 333–336.
- Ahmad, K.S. Remedial potential of bacterial and fungal strains (Bacillus subtilis, Aspergillus niger, Aspergillus flavus and Penicillium chrysogenum) against organochlorine insecticide Endosulfan. Folia Microbiol. 2020, 65, 801–810.
- Goswami, S.; Vig, K.; Singh, D.K. Biodegradation of α and β endosulfan by Aspergillus sydoni. Chemosphere 2009, 75, 883–888.
- Silambarasan, S.; Abraham, J. Mycoremediation of Endosulfan and Its Metabolites in Aqueous Medium and Soil by Botryosphaeria laricina JAS6 and Aspergillus tamarii JAS9. PLoS ONE 2013, 8, e77170.
- Abraham, J.; Silambarasan, S. Biomineralization and formulation of endosulfan degrading bacterial and fungal consortiums. Pestic. Biochem. Physiol. 2014, 116, 24–31.
- Taşeli, B.K. Dehalogenation of Lindane by Penicillium camemberti. Bull. Environ. Contam. Toxicol. 2006, 77, 882–887.
- Kumaravel, S.; Praveen Kumar, P.; Vasuki, P. GC-MS study on microbial degradation of Lindane. Int. J. Appl. Chem. 2010, 6, 363–366.
- Birolli, W.G.; Yamamoto, K.Y.; de Oliveira, J.R.; Nitschke, M.; Seleghim, M.H.R.; Porto, A.L.M. Biotransformation of dieldrin by the marine fungus Penicillium miczynskii CBMAI 930. Biocatal. Agric. Biotechnol. 2015, 4, 39–43.
- Soares, P.R.S.; Birolli, W.G.; Ferreira, I.M.; Porto, A.L.M. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185.
- Alvarenga, N.; Birolli, W.G.; Seleghim, M.H.R.; Porto, A.L.M. Biodegradation of methyl parathion by whole cells of marine-derived fungi Aspergillus sydowii and Penicillium decaturense. Chemosphere 2014, 117, 47–52.
- Marinho, G.; Rodrigues, K.; Araujo, R.; Pinheiro, Z.B.; Silva, G.M.M. Glucose effect on degradation kinetics of methyl parathion by filamentous fungi species Aspergillus niger AN400. Eng. Sanit. Ambient. 2011, 16, 225–230.
- Rodrigues, G.N.; Alvarenga, N.; Vacondio, B.; de Vasconcellos, S.P.; Passarini, M.R.Z.; Seleghim, M.H.R.; Porto, A.L.M. Biotransformation of methyl parathion by marine-derived fungi isolated from ascidian Didemnum ligulum. Biocatal. Agric. Biotechnol. 2016, 7, 24–30.
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Evaluating a preparation of malathion-tolerant Aspergillus niger MRU01 for accelerated removal of four organophosphorus insecticides. J. Appl. Chem. Biotechnol. 2021, 96, 1603–1610.
- Derbalah, A.; Khattab, I.; Saad Allah, M. Isolation and molecular identification of Aspergillus flavus and the study of its potential for malathion biodegradation in water. World J. Microbiol. Biotechnol. 2020, 36, 1–11.
- Yadav, M.; Srivastva, N.; Shukla, A.K.; Singh, R.S.; Upadhyay, S.N.; Dubey, S.K. Efficacy of Aspergillus sp. for degradation of chlorpyrifos in batch and continuous aerated packed bed bioreactors. Appl. Biochem. Biotechnol. 2015, 175, 16–24.
- Anggreini, C.D.; Tazkiaturrizki, T.; Rinanti, A. Chlorpyrifos removal improvement in liquid media by Aspergillus fumigatus. Int. J. Sci. Technol. Res. 2020, 9, 1475–1479.
- Anggreini, C.D.; Tazkiaturrizki, T.; Rinanti, A. The effect of temperature and concentration of Aspergillus fumigatus on chlorpyrifos removal. J. Phys. Conf. Ser. 2019, 1402, 033004.
- Silambarasan, S.; Abraham, J. Ecofriendly Method for Bioremediation of Chlorpyrifos from Agricultural Soil by Novel Fungus Aspergillus terreus JAS1. Water Air Soil Pollut. 2012, 224, 1369.
- Barberis, C.L.; Carranza, C.S.; Magnoli, K.; Benito, N.; Magnoli, C.E. Development and removal ability of non-toxigenic Aspergillus section Flavi in presence of atrazine, chlorpyrifos and endosulfan. Rev. Argent. Microbiol. 2019, 51, 3–11.
- Abdel-Wareth, M.T.A.; Abd El-Hamid, R.M. Mycoremediation of chlorpyrifos and lambda-cyhalothrin by two species of filamentous fungi. Int. J. Environ. Stud. 2016, 73, 974–987.
- Mukherjee, I.; Gopal, M. Degradation of chlorpyrifos by two soil fungi Aspergillus niger and Trichoderma viride. Toxicol. Environ. Chem. 1996, 57, 145–151.
- Abd-Alrahman, S.H.; Mostafa, A.A. Mycoremediation of organophosphorous insecticide chlorpyrifos by fungal soil isolates. J. Pure Appl. Microbiol. 2014, 8, 2945–2951.
- Karas, P.A.; Perruchon, C.; Exarhou, K.; Ehaliotis, C.; Karpouzas, D.G. Potential for bioremediation of agro-industrial effluents with high loads of pesticides by selected fungi. Biodegradation 2011, 22, 215–228.
- Omar, S.A. Availability of phosphorus and sulfur of insecticide origin by fungi. Biodegradation 1998, 9, 327–336.
- Zhao, R.B.; Bao, H.Y.; Liu, Y.X. Isolation and Characterization of Penicillium oxalicum ZHJ6 for Biodegradation of Methamidophos. Agric. Sci. China 2010, 9, 695–703.
- Fu, G.M.; Chen, Y.; Li, R.Y.; Yuan, X.Q.; Liu, C.M.; Li, B.; Wan, Y. Pathway and rate-limiting step of glyphosate degradation by Aspergillus oryzae A-F02. Prep. Biochem. Biotechnol. 2017, 47, 782–788.
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410.
- Derbalah, A.S.H.; Belal, E.B.; Massoud, A.H. Biodegradability of famoxadone by various microbial isolates in aquatic systems. Land Contam. Reclam. 2008, 16, 13–23.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
969
Revisions:
2 times
(View History)
Update Date:
10 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




