Since filamentous fungi of Penicillium and Aspergillus genera can colonize very diverse niches, and Ascomycota seems to be the dominant phylum within the microbial group in various contaminated substrates, they possess great potential in the remediation of pesticide-contaminated sites. Different species can remove the pesticides at different rates, and to various extents; however, the fungal ability to resist high concentrations of pesticides is almost unparalleled compared to other microbial groups. Their performance may be further improved by applying indigenous strains isolated from pesticide-contaminated soils and sediments.
- biotransformation
- filamentous fungi
- organochlorine
- organophosphorus pesticides
1. Introduction
2. Degradation of Organochlorine Pesticides by Aspergillus and Penicillium Species
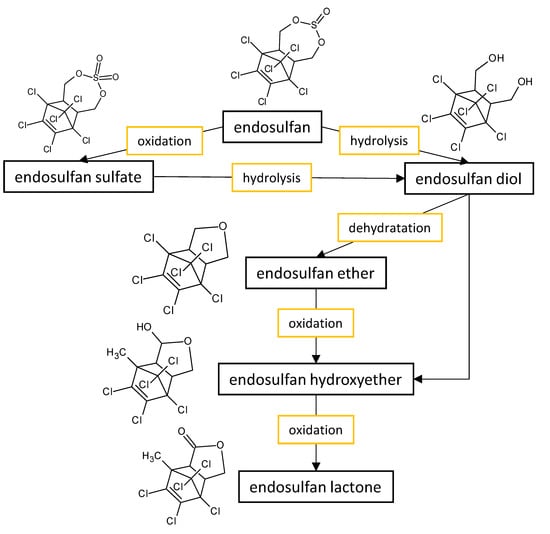
| Fungal Strain | Degradation Efficiency | Cultivation Conditions | Degradation Products | Reference |
|---|---|---|---|---|
| Penicillium sp. CHE23 | 94.8% | modified mineral salt medium with ~57 mg·L−1 of endosulfan as carbon source incubated for 144 h at 30 °C and 150 rpm | not reported | Romero-Aguilar et al. [31] |
| Aspergillus niger | complete degradation | Czapek–Dox broth with 350 mg·L−1 of β-endosulfan source incubated for 120 h at 30 °C and 120 rpm | endosulfan sulfate being most persistent metabolite; after 120 h, complete mineralization was suggested | Bhalerao and Puranik [32] |
| Aspergillus niger ARIFCC 1053 | complete degradation | Czapek–Dox broth with 1000 mg·L−1 of technical-grade endosulfan incubated for 7 days at 30 °C and 120 rpm | complete mineralization | Tejomyee [33] |
| Aspergillus niger | 98.6% | Czapek–Dox broth with 15.4 µg·g−1 of technical-grade endosulfan incubated for 15 days at 30 °C and intermittent shaking | not reported | Mukherjee and Gopal [34] |
| Aspergillus terreus, (Cladosporium oxysporum) |
91.5%, (89%) |
potato dextrose broth with 1.89 µg·g−1 of technical-grade endosulfan incubated for 15 days at 25 °C | trace concentrations of endosulfan sulfate were detected during incubation; no end products were reported | Mukherjee and Mittal [35] |
| Aspergillus terricola, Aspergillus terreus, (Chaetosartorya stromatoides) |
~90% | non-sulfur medium enriched with 100 mg·L−1 of α or β endosulfan isomers incubated for 12 days at 30 °C and 150 rpm (pH 6) | major metabolic products being endosulfan diol and endosulfan ether | Hussain et al. [36] |
| Aspergillus niger AE | complete degradation (0.1% endosulfan), and 76% (0.5% endosulfan) | Czapek–Dox broth spiked up to 5000 mg·L−1 (0.5%) of endosulfan incubated for 8 days at 30 °C and 180 rpm (optimum at pH 4) | not reported | Mukhtar et al. [37] |
| Aspergillus niger, Aspergillus flavus, Penicillium chrysogenum |
77% (AN), 72% (AF), 69% (PC) |
potato dextrose broth with 10 mg·L−1 of endosulfan incubated for 35 days at 29 °C | desulphurized transformants of endosulfan, while chlorine atoms remained imperforated | Ahmad [38] |
| Aspergillus sydoni | 95% (α isomer), 97% (β isomer) |
Czapek–Dox broth with 100 mg·L−1 of α or β endosulfan isomers incubated for 18 days at 30 °C and 150 rpm | major metabolic products being endosulfan sulfate | Goswami et al. [39] |
| Aspergillus tamarii JAS9, (Botryosphaerialaricina JAS6) |
kinetic analysis shows that 50% of α and β endosulfan isomers were degraded in 1.7 and 2.2 days by JAS9, respectively (4.2 days for 50% reduction of β endosulfan by JAS6) | M1 medium with 1000 mg·L−1 of technical-grade endosulfan as carbon source incubated for 10 days at 30 °C and 120 rpm | not reported | Silambarasan and Abraham [40] |
| fungal consortium (Botryosphaeria laricina JAS6, Aspergillus tamarii JAS9 and Lasiodiplodia sp. JAS12) | complete degradation (a 50% degradation calculated on the 3rd day of incubation) | M1 medium with 1000 mg·L−1 of technical-grade endosulfan as carbon source incubated for 120 h at 30 °C and 120 rpm | complete mineralization | Abraham and Silambarasan [41] |
| Penicillium camemberti | 70% | acetate-free basal medium supplemented with 1 mM lindane incubated for 120 h at 25 °C and 80 rpm (pH 5) | not reported | Taşeli [42] |
| Aspergillus fumigatus | complete degradation | lindane initial concentration is not indicated; medium (Sabouraud dextrose broth or Nutrient broth) incubated for 5 days at 25 °C | not reported | Kumaravel et al. [43] |
| Penicillium miczynskii CBMAI 93 | 90% | culture medium of artificial salt water supplemented with 50 mg·L−1 of dieldrin incubated for 14 days at 32 °C and 130 rpm | no intermediate degradation products were detected, suggesting dieldrin mineralization of conjugation | Birolli et al. [44] |
. Degradation of Organophosphorus Pesticides by Aspergillus and Penicillium Species
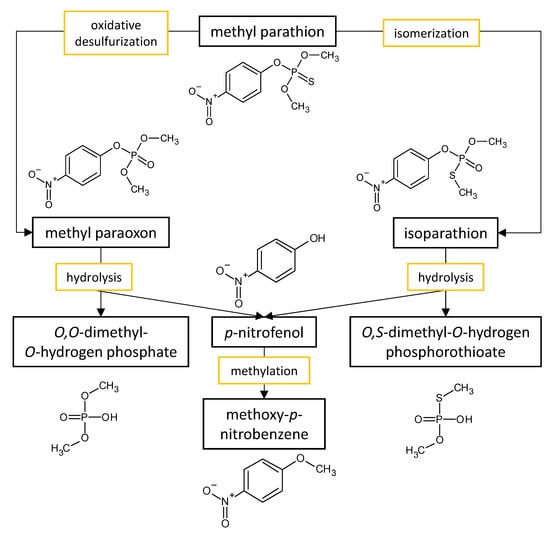
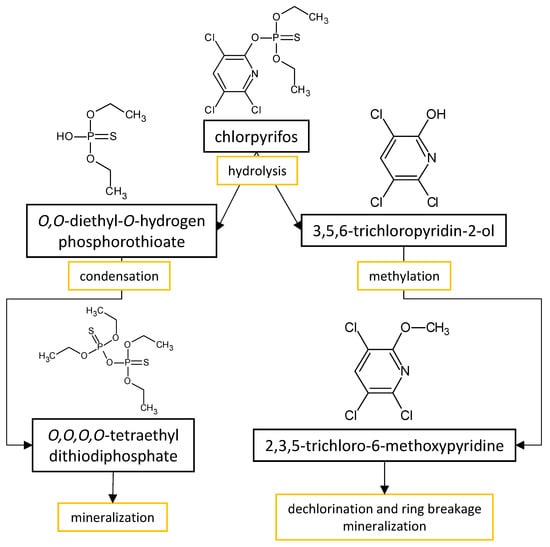
| Fungal Strain | Degradation Efficiency | Cultivation Conditions | Degradation Products | Reference |
|---|---|---|---|---|
| Aspergillus sydowii CBMAI 935 | 32%, 80% and 52% of chlorpyrifos, methyl parathion, and profenofos, respectively | 2% malt liquid medium with 50 mg·L−1 of chlorpyrifos, methyl parathion or profenofos incubated for 30 days at 32 °C and 130 rpm | chlorpyrifos degradation resulted in tetraethyl dithiodi- phosphate and 2,3,5-trichloro-6-methoxypyridine; methyl parathion hydrloyzed into methylated phosphate and phosphorothioates, and 1-methoxy-4-nitrobenzen; profenofos degraded into 4-bromo-2-chloro-1-methoxybenzen and O,O-diethyl-S-proyl phosphorothioates |
Soares et al. [50] |
| Aspergillus sydowii CBMAI 935, Penicillium decaturense CBMAI 1234 |
80% | liquid mineral medium supplemented with KNO3 and 100 mg·L−1 of methyl parathion incubated for 30 days at 32 °C and 130 rpm | p-nitrophenol | Alvarenga et al. [51] |
| Aspergillus niger AN400 | 2% (glucose-free treatment, initial concentration of methyl parathion was 19.1 mg·L−1), 43% (glucose-treated medium, initial concentration of methyl parathion 24.9 mg·L−1) |
glucose-free or glucose treated distilled water supplemented with Vishniac solution and up to 24.9 mg·L−1 of methyl parathion incubated for 27 days at 30 °C and 200 rpm | not reported | Marinho et al. [52] |
| Penicillium citrinum, (Fusarium proliferatum) |
complete degradation (the biotic control had the same degradation efficiency) | 3% malt liquid medium with 30 mg·L−1 of methyl parathion incubated for 30 days at 32 °C and 130 rpm | not reported | Rodrigues et al. [53] |
| Aspergillus niger MRU01 | 70%, 54%, 58%, and 68% of malathion, parathion, chlorpyrifos and dimethoate, respectively | Czapek–Dox broth spiked with 500, 470, 260 and 680 μmol·L−1 (0.5%) of malathion, parathion, chlorpyrifos, and dimethoate, respectively, incubated for 5 days at 26 °C and 120 rpm (optimum at pH 4) | not reported | Mohapatra et al. [54] |
| Aspergillus flavus | complete degradation | mineral salt medium supplemented with 5 mg·L−1 of malathion incubated for 36 days at 30 °C (pH 7) on a rotatory shaker (optimized conditions) | not reported | Derbalah et al. [55] |
| Aspergillus sp. F1 | over 90% (89% at an inlet load of 180 mg·L−1·d−1) |
bioreactor supplemented with 300 mg·L−1 of chlorpyrifos as the sole carbon source incubated at 28 °C (pH 7) with dissolved oxygen concentration of 5.8 mg·L−1 (optimized conditions) | not reported | Yadav et al. [56] |
| Aspergillus fumigatus | 99% | potato dextrose broth supplemented with chlorpyrifos (10%) incubated for 9 days at 25 °C (pH 7) and 180 rpm | not reported | Anggreini et al. [57] |
| Aspergillus fumigatus | 95.9% | potato dextrose broth with chlorpyrifos (1.5%) incubated for 5 days at 25 °C (pH 7) and 180 rpm | not reported | Anggreini et al. [58] |
| Aspergillus terreus JAS1 | complete degradation (after 24 h) | M1 medium supplemented with 300 mg·L−1 of chlorpyrifos as the sole carbon source incubated for 96 h at 30 °C and 120 rpm | 3,5,6-trichloropyridin-2-ol that was completely degraded after 48 h; no other metabolites were reported | Silambarasan and Abraham [59] |
| Aspergillus oryzae strains AM1 and AM2 | 73% (AM1), 50% (AM2) |
Czapek–Dox broth spiked with 20 mg·L−1 of chlorpyrifos incubated for 30 days at 25 °C and 60 rpm (optimum at pH 4) | not reported | Barberis et al. [60] |
| Aspergillus viridinutans, Penicillium implicatum | 44.6% (A. viridinutans), 16.2% (P. implicatum) |
potato dextrose broth with 20 mg·L−1 of chlorpyrifos incubated for 14 days at 28 °C | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Abdel-Wareth and Abd El-Hamid [61] |
| Aspergillus niger, (Trichoderma viride) |
72.3%, (95.7%) |
Czapek–Dox broth spiked with 1.25 mg·L−1 of chlorpyrifos incubated for 14 days at 30 °C and intermittent shaking (pH 6.8) | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Mukherjee and Gopal [62] |
| Penicillium citrinum, Aspergillus niger, Aspergillus oryzae |
25.9% (P. citrinum), 64% (A. niger), 50.8% (A. oryzae) | Burkes mineral broth with 10 mg·L−1 of chlorpyrifos incubated for 15 days at 27 °C without shaking (pH 7.2) | not reported; high losses of chlorpyrifos from culture medium were due to abiotic hydrolysis |
Abd-Alrahman and Mostafa [63] |
| Aspergillus niger | ~80% | soil extract medium with 10 mg·L−1 of chlorpyrifos incubated for 30 days at 25 °C and 60 rpm | 3,5,6-trichloro-2-pyridinol were detected below the concentration of 1 mg·L−1 | Karas et al. [22] |
| Aspergillus fumigatus, A. flavus, A. niger, A. ochraceus, A. tamarii, A. terreus, Penicillium chrysogenum, P. brevicompactum, P. citrinum, P. funiculosum | phosphor mineralization efficiencies ranged from 4 to 46% (Cyolan), from 9.5 to 26.8% (Malathion), and from 2.3 to 6.7% (Dursban) | Czapek–Dox broth spiked with 100 mg·L−1 of cyolan, malathion, and chlorpyrifos incubated for 35 days at 28 °C without shaking | not reported; media and biomass were analyzed for phosphor and sulfur that mineralized from the degradation of insecticide |
Omar [64] |
| Penicillium oxalicum ZHJ6 | complete degradation | mineral salt medium with 1% glucose supplemented with 1 mg·L−1 of methamidophos as sole nitrogen source incubated for 12 days at 25 °C and pH of 5.0 (most favorable conditions) | inorganic phosphor, CH3SH, and CH3OH are hypothesized being formed | Zhao et al. [65] |
| Aspergillus oryzae A-F02 | ~13.7% | fermentation medium with 1.5 g·L−1 of glyphosate incubated for 144 h at 30 °C and 150 rpm | aminomethylphosphonic acid and methylamine, the latter being further degraded | Fu et al. [66] |
4. Biotransformation of Pesticide Belonging to Some Other Chemical Groups by Aspergillus and Penicillium Species
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11061485
