
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samy Emara | -- | 2555 | 2023-07-03 13:53:13 | | | |
| 2 | Conner Chen | Meta information modification | 2555 | 2023-07-04 04:51:32 | | |
Video Upload Options
Despite the fact that strong routine separation methodologies can give reliable specificity and validity at usual working pharmaceutical concentrations, they may fail at very low concentration levels. This poses considerable challenges for researchers investigating product purity and therapeutic drug monitoring. Sensitivity enhancement procedures are thus required to maximize the performance of separation techniques. Solid-phase extraction/solid-phase enrichment (SPE/SPEn) and pre-, post-, and in-column derivatization, as well as the use of sensitive detection devices, are the simplest strategies for improving sensitivity of separation-based analytical techniques. Large-volume injection of samples with online SPE/SPEn coupled with separation techniques increased sensitivity and improved detection as well as quantification limits without affecting peak shape and system performance.
1. Introduction
2. Solid-Phase Extraction/Pre-Concentration Strategies for Drug Analysis
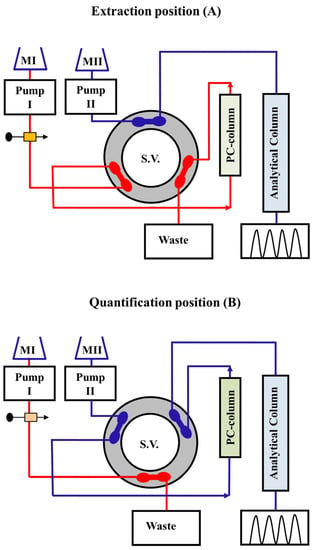
2.1. Offline Solid-Phase Extraction/Enrichment
2.2. Offline Solid-Phase Microextraction
| Analyte | Sample Matrix | Separation Technique | Detection System | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|
|
Water | HPLC | UV-Vis | 0.50 ppm 0.09 ppm 0.09 ppm 1.48 ppm 0.65 ppm |
1.69 ppm 0.32 ppm 0.32 ppm 4.96 ppm 2.19 ppm |
[25] |
|
Plasma | HPLC | UV-Vis | - | 1.25 ng/mL 1.75 ng/mL |
[26] |
|
Plasma | HPLC | UV-Vis | 1 ng/mL 1 ng/mL |
2 ng/mL 2 ng/mL |
[27] |
|
Milk | HPLC | DAD | 18 lg/kg | 60 lg/kg | [28] |
|
Water | HPLC | UV-Vis | 0.1 ng/mL | 0.5 ng/mL | [30] |
|
Milk formula | HPLC | UV-Vis | 0.01 µg/mL | 0.033 µg/mL | [31] |
|
Urine | HPLC | MS/MS | 0.015 ng/mL 0.015 ng/mL 0.015 ng/mL 0.015 ng/mL |
0.025 ng/mL 0.025 ng/mL 0.020 ng/mL 0.030 ng/mL |
[37] |
|
Urine | HPLC | Fluorescence | 0.01 µg/mL 0.02 µg/mL 0.1 µg/mL 0.001 µg/mL 0.05 µg/mL |
1 µg/mL 0.05 µg/mL 0.3 µg/mL 0.003 µg/mL 0.25 µg/mL |
[43] |
|
Plasma | HPLC | UV-Vis | - | 75 ng/mL 75 ng/mL 75 ng/mL 75 ng/mL 5 ng/mL 6 ng/mL 5 ng/mL 75 ng/mL |
[44] |
|
Urine | UHPLC | MS/MS | 0.0125 ng/mL | 0.1 ng/mL | [45] |
|
Plasma | UPLC | MS/MS | 0.04 µg/mL 01 µg/mL 0.02 µg/mL |
0.15 ug/mL 0.32 ug/mL 0.06 ug/mL |
[46] |
|
Plasma | HPLC | MS/MS | - | 50.2 ng/mL 1.25 ng/mL |
[47] |
|
Wastewater | UPLC | MS/MS | 0.02 ng/mL | 0.05 ng/mL | [48] |
|
Saliva | UPLC | DAD | 3 ng/mL | 5 ng/mL | [49] |
|
Wastewater | CE | UV-Vis | 3 µg/mL 3 µg/mL 3 µg/mL 3 µg/mL |
5 µg/mL 5 µg/mL 5 µg/mL 5 µg/mL |
[50] |
|
Chicken feces | HPLC | DAD | 0.14 mg/mL 0.14 mg/mL |
0.45 mg/mL 0.43 mg/mL |
[51] |
|
Urine | CE | MS/MS | 1 ng/mL 1 ng/mL 0.60 ng/mL |
5 ng/mL 8 ng/mL 2 ng/mL |
[52] |
|
Water | CE | DAD | 0.016 µg/mL 0.040 µg/mL 0.097 µg/mL 0.037 µg/mL 0.037 µg/mL |
0.05 µg/mL 0.14 µg/mL 0.33 µg/mL 0.13 µg/mL 0.13 µg/mL |
[53] |
|
Tea infusions | CE | Conductivity | 0.80 ng/mL 0.56 ng/mL 1.56 ng/mL 0.54 ng/mL |
2.68 ng/mL 1.87 ng/mL 5.19 ng/mL 1.82 ng/mL |
[54] |
|
Milk | CE | UV-Vis | 19.93 ng/mL 23.83 ng/mL 18.60 ng/mL |
59.79 ng/mL 71.49 ng/mL 55.8 ng/mL |
[55] |
|
Milk | CE | UV-Vis | 0.16 µg/mL 0.04 µg/mL 0.03 µg/mL 0.10 µg/mL 0.07 µg/mL 0.03 µg/mL 0.20 µg/mL 0.07 µg/mL |
0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL |
[56] |
|
Plasma | GC | MS/MS | 0.5 ng/mL | 1 ng/mL | [57] |
|
Foods | CE | UV-Vis | 0.087 ng/mL | 0.29 ng/mL | [58] |
References
- Aydin, D.C.; Pineres, J.Z.; Al-Manji, F.; Rijnaarts, H.; Grotenhuis, T. Direct Analysis of Aromatic Pollutants Using an HPLC-FLD/DAD Method for Monitoring Biodegradation Processes. Anal. Methods 2021, 13, 1635–1642.
- Chiriac, U.; Rau, H.; Frey, O.R.; Röhr, A.C.; Klein, S.; Meyer, A.L.; Morath, B. Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Dalbavancin. Antibiotics 2022, 11, 541.
- Al-Sanea, M.M.; Gamal, M. Critical Analytical Review: Rare and Recent Applications of Refractive Index Detector in HPLC Chromatographic Drug Analysis. Microchem. J. 2022, 178, 107339.
- Marzouk, H.M.; Rezk, M.R.; Gouda, A.S.; Abdel-Megied, A.M. A Novel Stability-Indicating HPLC-DAD Method for Determination of Favipiravir, a Potential Antiviral Drug for COVID-19 Treatment; Application to Degradation Kinetic Studies and In-Vitro Dissolution Profiling. Microchem. J. 2022, 172, 106917.
- El-Gindy, A.; Emara, S.; Mesbah, M.K.; Hadad, G.M. Liquid Chromatography Determination of Citalopram Enantiomers Using Beta-Cyclodextrin as a Chiral Mobile Phase Additive. J. AOAC Int. 2006, 89, 65–70.
- El-Gindy, A.; Emara, S.; Mesbah, M.K.; Hadad, G.M. Liquid Chromatography and Chemometric-Assisted Spectrophotometric Methods for the Analysis of Two Multicomponent Mixtures Containing Cough Suppressant Drugs. J. AOAC Int. 2005, 88, 1069–1080.
- Hadad, G.M.; Emara, S.; Mahmoud, W.M.M. Optimization and Validation of an LC Method for the Determination of Cefdinir in Dosage form and Human Urine. Chromatographia 2009, 70, 1593–1598.
- EL-Gindy, A.; Emara, S.; Shaaban, H. Stability-Indicating Method for Determination of Oxyphenonium Bromide and Its Degradation Product by High-Performance Liquid Chromatography. J. AOAC Int. 2007, 90, 1250–1257.
- Mostafa, A.; El-Gindy, A.; Emara, S. Development, Application and Validation of RP-HPLC Method for the Simultaneous Determination of Butamirate Citrate and its Main Degradation Product in Pharmaceutical Dosage forms. Anal. Methods 2011, 3, 1643–1651.
- El-Gindy, A.; Emara, S.; Mostafa, A. HPLC and Chemometric-Assisted Spectrophotometric Methods for Simultaneous Determination of Atenolol, Amiloride Hydrochloride and Chlorthalidone. Il Farm. 2005, 60, 269–278.
- Hadad, G.M.; Salam, R.A.A.; Emara, S. Validated Stability—Indicating HPTLC and HPLC Methods for Determination of Pipazethate and its Degradant. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1850–1869.
- Hadad, G.M.; Emara, S.; Mahmoud, W.M.M. Development and Validation of a Stability-Indicating RP-HPLC Method for the Determination of Paracetamol with Dantrolene or/and Cetirizine and Pseudoephedrine in Two Pharmaceutical Dosage forms. Talanta 2009, 79, 1360–1367.
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106.
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180.
- Oliveira, E.d. Sample Preparation for Atomic Spectroscopy: Evolution and Future Trends. J. Braz. Chem. Soc. 2003, 14, 174–182.
- Urge, A.Y.; Pampanin, D.M.; Martino, M.E.; Knudsen, D.L.; Brede, C. Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma. Separations 2023, 10, 240.
- Zygler, A.; Wasik, A.; Namieśnik, J. Retention Behaviour of Some High-Intensity Sweeteners on Different SPE Sorbents. Talanta 2010, 82, 1742–1748.
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. Review of the Modern Principles and Applications of Solid-Phase Extraction Techniques in Chromatographic Analysis. Anal. Sci. 2022, 38, 1457–1487.
- Hadad, G.M.; Abdel Salam, R.A.; Emara, S. Validated and Optimized High-Performance Liquid Chromatographic Determination of Tizoxanide, the Main Active Metabolite of Nitazoxanide in Human Urine, Plasma and Breast Milk. J. Chromatogr. Sci. 2012, 50, 509–515.
- Ebrahim, H.; Sonbol, H.; Malak, M.; Ali, A.; Aboulella, Y.; Hadad, G.; Zarad, W.; Emara, S.; Bazan, L. Green Automated Solid Phase Extraction to Measure Levofloxacin in Human Serum via Liquid Chromatography with Fluorescence Detection for Pharmacokinetic Study. Separations 2023, 10, 136.
- Sonbol, H.; Ebrahim, H.; Malak, M.; Ali, A.; Aboulella, Y.; Hadad, G.; Emara, S.; Shawky, A. Application of a Small Protein-Coated Column to Trap, Extract and Enrich Carbamazepine Directly from Human Serum for Direct Chromatographic Analysis. Separations 2023, 10, 71.
- Abughrin, S.E.; Alshana, U.; Bakirdere, S. Magnetic Nanoparticle-Based Dispersive Solid-Phase Microextraction of Three UV Blockers Prior to their Determination by HPLC-DAD. Int. J. Environ. Res. Public Health 2022, 19, 6037.
- Nishida, M.; Namera, A.; Yashiki, M.; Kojima, T. On-Column Derivatization for Determination of Amphetamine and Methamphetamine in Human Blood by Gas Chromatography–Mass Spectrometry. Forensic Sci. Int. 2002, 125, 156–162.
- Malak, M.; Ebrahim, H.; Sonbol, H.; Ali, A.; Aboulella, Y.; Hadad, G.; Emara, S. Highly Sensitive In-Capillary Derivatization and Field Amplified Sample Stacking to Analyze Narcotic Drugs in Human Serum by Capillary Zone Electrophoresis. Separations 2023, 10, 58.
- Archana, G.; Dhodapkar, R.; Kumar, A. Offline Solid-Phase Extraction for Preconcentration of Pharmaceuticals and Personal Care Products in Environmental Water and their Simultaneous Determination Using the Reversed Phase High-Performance Liquid Chromatography Method. Environ. Monit. Assess. 2016, 188, 512.
- Dawson, R.; Messina, S.M.; Stokes, C.; Salyani, S.; Alcalay, N.; De Fiebre, N.C.; De Fiebre, C.M. Solid-Phase Extraction and HPLC Assay of Nicotine and Cotinine in Plasma and Brain. Toxicol. Mech. Methods 2002, 12, 45–58.
- Torres, V.P.; Sepúlveda, C.M.J.; Von Plessing, R.C. Pharmacokinetic Study of Risperidone: Application of an HPLC Method with Solid Phase Extraction. J. Chil. Chem. Soc. 2011, 56, 606–609.
- Sun, H.; Wang, L.; Ai, L.; Liang, S.; Wu, H. A Sensitive and Validated Method for Determination of Melamine Residue in Liquid Milk by Reversed Phase High-Performance Liquid Chromatography with Solid-Phase Extraction. Food Control 2010, 21, 686–691.
- Xu, Y.; Chen, L.; Wang, H.; Zhang, X.; Zeng, Q.; Xu, H.; Sun, L.; Zhao, Q.; Ding, L. Preparation of Magnetic Strong Cation Exchange Resin for the Extraction of Melamine from Egg Samples Followed by Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chim. Acta 2010, 661, 35–41.
- He, L.; Su, Y.; Shen, X.; Zheng, Y.; Guo, H.; Zeng, Z. Solid-Phase Extraction of Melamine from Aqueous Samples Using Water-Compatible Molecularly Imprinted Polymers. J. Sep. Sci. 2009, 32, 3310–3318.
- Wang, T.; Zhu, Y.; Ma, J.; Xuan, R.; Gao, H.; Liang, Z.; Zhang, L.; Zhang, Y. Hydrophilic Solid-Phase Extraction of Melamine with Ampholine-Modified Hybrid Organic–Inorganic Silica Material. J. Sep. Sci. 2015, 38, 87–92.
- Braus, H.; Middleton, F.; Walton, G. Organic Chemical Compounds in Raw and Filtered Surface Waters. Anal. Chem. 1951, 23, 1160–1164.
- Theodoridis, G.; Koster, E.H.M.; de Jong, G.J. Solid-Phase Microextraction for the Analysis of Biological Samples. J. Chromatogr. B 2000, 745, 49–82.
- Zheng, J.; Huang, J.; Yang, Q.; Ni, C.; Xie, X.; Shi, Y.; Sun, J.; Zhu, F.; Ouyang, G. Fabrications of Novel Solid Phase Microextraction Fiber Coatings Based on New Materials for High Enrichment Capability. Trends Anal. Chem. 2018, 108, 135–153.
- Gao, Y.; Sheng, K.; Bao, T.; Wang, S. Recent Applications of Organic Molecule-Based Framework Porous Materials in Solid-Phase Microextraction for Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2022, 221, 115040.
- Arrebola, F.J.; Martínez-Vidal, J.L.; Fernández-Gutiérrez, A.; Akhtar, M.H. Monitoring of Pyrethroid Metabolites in Human Urine Using Solid-Phase Extraction Followed by Gas Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 1999, 401, 45–54.
- Le Grand, R.; Dulaurent, S.; Gaulier, J.M.; Saint-Marcoux, F.; Moesch, C.; Lachâtre, G. Simultaneous Determination of Five Synthetic Pyrethroid Metabolites in Urine by Liquid Chromatography–Tandem Mass Spectrometry: Application to 39 Persons without Known Exposure to Pyrethroids. Toxicol. Lett. 2012, 210, 248–253.
- Wielgomas, B.; Nahorski, W.; Czarnowski, W. Urinary Concentrations of Pyrethroid Metabolites in the Convenience Sample ofan Urban Population of Northern Poland. Int. J. Hyg. Environ. Health 2013, 216, 295–300.
- Lin, C.-H.; Yan, C.-T.; Kumar, P.V.; Li, H.-P.; Jen, J.-F. Determination of Pyrethroid Metabolites in Human Urine Using Liquid Phase Microextraction Coupled In-Syringe Derivatization Followed by Gas Chromatography/Electron Capture Detection. Anal. Bioanal. Chem. 2011, 401, 927–937.
- Bartosz, W.; Marcin, W.; Wojciech, C. Development of Hollow Fiber-Supported Liquid-Phase Microextraction and HPLC-DAD Method for the Determination of Pyrethroid Metabolites in Human and Rat Urine. Biomed. Chromatogr. 2014, 28, 708–716.
- Klimowska, A.; Wielgomas, B. Off-Line Microextraction by Packed Sorbent Combined with on Solid Support Derivatization and GC-MS: Application for the Analysis of Five Pyrethroid Metabolites in Urine Samples. Talanta 2018, 176, 165–171.
- Bernardo, R.A.; da Silva, L.C.; Queiroz, M.E.C.; Vaz, B.G.; Chaves, A.R. Lab-Made Solid Phase Microextraction Phases for off Line Extraction and Direct Mass Spectrometry Analysis: Evaluating the Extraction Parameters. J. Chromatogr. A 2019, 1603, 23–32.
- Theodoridis, G.; Lontou, M.A.; Michopoulos, F.; Sucha, M.; Gondova, T. Study of Multiple Solid-Phase Microextraction Combined Off-Line with High Performance Liquid Chromatography: Application in the Analysis of Pharmaceuticals in Urine. Anal. Chim. Acta 2004, 516, 197–204.
- Cantú, M.D.; Toso, D.R.; Lacerda, C.A.; Lanças, F.M.; Carrilho, E.; Queiroz, M.E.C. Optimization of Solid-Phase Microextraction Procedures for the Determination of Tricyclic Antidepressants and Anticonvulsants in Plasma Samples by Liquid Chromatography. Anal. Bioanal. Chem. 2006, 386, 256–263.
- Slíž, K.; Olešová, D.; Piešt’anský, J.; Mikuš, P. Simple and Sensitive Analysis of Clenbuterol in Urine Matrices by UHPLC-MS/MS Method with Online-SPE Sample Preparation. Separations 2022, 9, 440.
- Kovačić, J.; Jeličić, M.-L.; Klarić, D.A.; Mornar, A. Green Solid-Phase (Micro)Extraction of Andrographolides from Human Plasma Samples Followed by UHPLC-DAD-QqQ-MS/MS Analysis. Separations 2023, 10, 69.
- Haque, A.; Iqbal, M.; Alamoudi, M.K.; Alam, P. A Selective and Accurate LC-MS/MS Method for Simultaneous Quantification of Valsartan and Hydrochlorothiazide in Human Plasma. Separations 2023, 10, 119.
- Haq, N.; Iqbal, M.; Hussain, A.; Shakeel, F.; Ahmad, A.; Ibrahim, A.; Alsarra, I.A.; AlAjmi, M.F.; Mahfooz, A.; Abouzadeh, M.A. Utilization of Waste Biomaterial as an Efficient and Eco-Friendly Adsorbent for Solid-Phase Extraction of Pantoprazole Contaminants in Wastewater. Separations 2023, 10, 253.
- Dziurkowska, E.; Kosinska, S.; Plenis, A.; Wesolowski, M. A New Method for the Determination of Amisulpride in a Small Volume (200 µL) of Human Saliva Using LC-DAD Supported by SPE. Separations 2023, 10, 277.
- Abdel-Gawad, S.A.; Altharawi, A. Simultaneous Quantification of Some Fluoroquinolone Residues in Real Wastewater Effluents Using CZE. Separations 2023, 10, 292.
- Díaz-Corona, L.R.; Parra-Saavedra, K.J.; Mora-Alonzo, R.S.; Macías-Rodríguez, M.E.; Martínez-Preciado, A.H.; Guevara-Martínez, S.J.; Zamudio-Ojeda, A.; Macias-Lamas, A.M. HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations 2023, 10, 308.
- Pascual-Caro, S.; Borrull, F.; Aguilar, C.; Calull, M. Comparison of different chiral selectors for the enantiomeric determination of amphetamine-type substances in human urine by solid-phase extraction followed by capillary electrophoresis-tandem mass spectrometry. Electrophoresis 2022, 43, 437–445.
- Yang, J.; Chen, L.; Wang, Q.; Mei, X.; Yang, X.; Huo, F. Determination of nitroimidazole antibiotics based on dispersive solid-phase extraction combined with capillary electrophoresis. Electrophoresis 2023, 44, 634–645.
- Nguyen, M.H.; Nguyen, T.D.; Vu, M.T.; Duong, H.A.; Pham, H.V. Determination of Glyphosate, Glufosinate, and Their Major Metabolites in Tea Infusions by Dual-Channel Capillary Electrophoresis following Solid-Phase Extraction. J. Anal. Methods Chem. 2022, 2022, 5687025.
- Islas, G.; Rodriguez, J.A.; Perez-Silva, I.; Jose, M.; Miranda, J.M.; Ibarra, I.S. Solid-Phase Extraction and Large-Volume Sample Stacking-Capillary Electrophoresis for Determination of Tetracycline Residues in Milk. J. Anal. Methods Chem. 2018, 2018, 5394527.
- An, J.; Wang, X.; Ming, M.; Li, J.; Ye, N. Determination of sulfonamides in milk by capillary electrophoresis with 2 as a dispersive solid-phase extraction sorbent. R. Soc. Open Sci. 2018, 5, 172104.
- Chik, Z.; Mustafa, A.M.; Mohamed, Z.; Lee, T.C. Analysis of Captopril in Human Plasma Using Gas Chromatography-Mass Spectrometry (GCMS) with Solid-Phase Extraction (SPE). Curr. Anal. Chem. 2010, 6, 329–333.
- Fan, Y.; Yu, R.; Chen, Y.; Sun, Y.; Waterhouse, G.I.N.; Xu, Z. A Capillary Electrophoresis Method Based on Molecularly Imprinted Solid-Phase Extraction for Selective and Sensitive Detection of Histamine in Foods. Molecules 2022, 27, 6987.




