Despite the fact that strong routine separation methodologies can give reliable specificity and validity at usual working pharmaceutical concentrations, they may fail at very low concentration levels. This poses considerable challenges for researchers investigating product purity and therapeutic drug monitoring. Sensitivity enhancement procedures are thus required to maximize the performance of separation techniques. Solid-phase extraction/solid-phase enrichment (SPE/SPEn) and pre-, post-, and in-column derivatization, as well as the use of sensitive detection devices, are the simplest strategies for improving sensitivity of separation-based analytical techniques. Large-volume injection of samples with online SPE/SPEn coupled with separation techniques increased sensitivity and improved detection as well as quantification limits without affecting peak shape and system performance.
- separation-based analytical techniques
- large-volume injection
- solid-phase enrichment
1. Introduction
2. Solid-Phase Extraction/Pre-Concentration Strategies for Drug Analysis
Because of the lower concentration levels of pharmaceuticals and the high levels of interferences present in bio-fluid samples, sample clean-up and enrichment processes are critical prior to chromatographic analysis to optimize technique sensitivity, recovery, and accuracy. It is difficult to justify extraction procedures that employ large amounts of hazardous organic solvents in the sample preparation steps in an era when it is recommended to implement green chemistry principles in analytical laboratories. Of the different purification methods, SPE is a quick, low-cost, and extensively applicable technology. Furthermore, it is broadly applicable for the enrichment of pharmaceuticals and extraction of biological interferences with high removal effectiveness. SPE is seen as a good substitute for LLE because it moves past many of the shortcomings of LLE [16][17][18][16,17,18]. Moreover, the entire procedure can be automated. Besides that, SPE does not require phase separation, as LLE does, which eliminates errors related to inaccurately estimated extract volumes, one of the primary reasons for errors observed in the analysis of extracts obtained by LLE. Thus, major efforts have been made to design and evaluate innovative formats and efficient sorbent materials in order to improve their selectivity, specificity, and sorption capacity towards target analytes and enhance physicochemical or mechanical stability, among other SPE-related properties. Solventless sample preparation approaches based on analyte extraction and enrichment by online SPE, using phosphate buffers as washing solvents, have been proven to be viable and environmentally friendly alternatives to conventional solvent extraction techniques [20][21][20,21]. Fluconazole in serum has been directly quantified by trapping on a pre-column and subsequently separating on an analytical column, utilizing an elution mode by online SPE and HPLC methods (Figure 1) [21]. Because of its simplicity of use, flexibility, rapid extraction time, safety, minimal organic solvent consumption, and high enrichment factor, solid-phase microextraction (SPME) is a promising sample pretreatment approach [22]. To boost sensitivity in separation techniques, the large-volume injection approach and online sample pre-concentration have been widely employed. Highly sensitive and selective HPLC methodologies with less costly fluorescence (FL) detection systems are also required. Thus, the derivatization technique is necessary to enable sensitivity enhancement by converting non- or weakly native fluorescent compounds into highly fluorescence derivatives. The combination of pre- or post-column derivatization procedures with the chromatographic systems, as well as FL detection, made it possible for determining different medications at low concentration levels.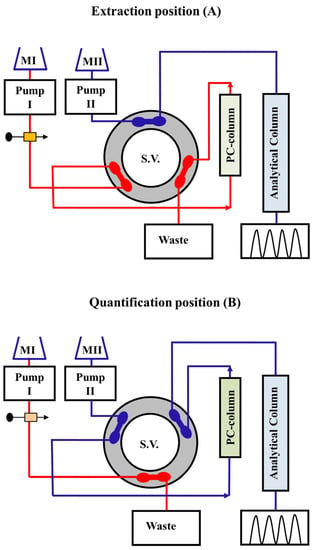
2.1. Offline Solid-Phase Extraction/Enrichment
Advanced sorbent technologies reorient SPE materials with various functionalities according to their structures, such as RP, normal-phase (NP), cation exchange (CEx), anion exchange (AEx), and mixed-mode types. Offline SPE in conjunction with the RP-HPLC approach for highly sensitive determination of ciprofloxacin, acetaminophen, caffeine, benzophenone, and irgasan in aquatic environments has been reported [25]. The SPE was conducted prior to the analysis using an RP-C18 cartridge to pre-concentrate the tested analytes from the ecological water samples. Dawson et al. reported a simple and reliable assay for nicotine and its main metabolite, cotinine, in plasma [26]. On the silica columns, an extraction/pre-concentration approach compatible with RP-HPLC separation was devised followed by quantification on ODS column using UV detection. Torre et al. simplified the SPE process for determining risperidone and 9-hydroxyrisperidone in human plasma using polymeric RP sorbents [27]. The SPE-HPLC approach for assessing melamine in liquid milk has been reported to meet the detection demand for melamine-contaminated milk [28]. The developed method was validated using LC tandem mass spectrometry (LC-MS/MS) and has been successfully applied for routine melamine measurement in a variety of milk samples. Moreover, the CEx resin column is utilized for separation and pre-concentration purposes in LC-MS to analyze melamine in egg samples [29]. He et al. and Wang et al. described rapid and efficient SPE procedures followed by HPLC-UV methods for the determination of melamine in aqueous and milk formula samples, respectively [30][31][30,31].2.2. Offline Solid-Phase Microextraction
The application of the twelve green chemistry principles to laboratory practice surely fueled the search for innovative methodological approaches to guarantee an improvement in results quality while enhancing environmental friendliness. Since the concept of SPE of target analytes was developed, substantial advancements in this technology have been noted, including the original concept’s simplification, automation, and miniaturization. The method put forth in 1951 by Braus and colleagues, which was based on the insertion of up to 1.2–1.5 kg of granular activated carbon into an iron cylinder, is fundamentally different from the SPE formats currently employed in laboratory practice [32]. Because of undeniable advances in both the adsorption process and the large-scale production of new classes of materials, practical solutions for achieving high rates of recovery and enrichment while using significantly less sorbents and organic solvents have become possible for trapping various types of analytes. SPME is presently gaining popularity in a variety of fields of investigation, including dietary, biological, and pharmaceutical products [33]. SPME provides several advantages, including ease of use, low cost, compatibility with analytical systems, automation, and a solventless extraction procedure. In recent years, SPME has been employed prior to LC and CE, in addition to its application with GC. Enrichments of pharmaceuticals from various samples with complex matrices make it necessary to produce unique SPME fiber coatings such as metal organic frameworks, covalent organic frameworks, carbon, polymer, ionic liquids, metal/metal oxide nanoparticles, and silica [34][35][34,35]. The majority of pyrethroid metabolites are extracted from urine in the literatures using SPE or LLE, before being subjected to GC-MS or LC-MS [36][37][38][36,37,38]. As a pretreatment procedure to meet the demands for rapid and environmentally friendly extraction protocols, SPME is becoming more important. There have been various SPME methods reported in the literature [39][40][39,40], but they are not sensitive enough to measure the trace concentrations of pyrethroid metabolites that are important for evaluating environmental exposure. For the analysis of pyrethroid metabolites in urine samples, a green analytical method by a packed sorbent coupled to large-volume injection and GC-MS has been developed using an offline SPME technique [41]. With the aid of direct MS technology, quantification of cocaine, methamphetamine, 3,4-Methyl-enedioxy-methamphetamine, and lysergic acid diethylamide from oral fluid and urine samples has been conducted using offline SPME [42]. Quinine, naproxen, haloperidol, ciprofloxacin, and paclitaxel have all been extracted using multiple SPME [43]. After SPME, desorption was carried out offline, each drug was then analyzed using HPLC with UV or FL detection. Cantú et al. reported an offline SPME technique for analyzing anticonvulsants and tricyclic antidepressants in human plasma for TDM purposes by HPLC-UV [44]. For measuring various medicines in several matrices using offline SPE, different separation-based analytical approaches have been proposed [45][46][47][48][49][50][51][52][53][54][55][56][57][58][45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Table 1 lists the LODs, LOQs, sample matrices, and offline extraction and separation processes as well as the detection systems used for the analysis of various pharmaceutics.| Analyte | Sample Matrix | Separation Technique | Detection System | LOD | LOQ | Ref. |
|---|---|---|---|---|---|---|
|
Water | HPLC | UV-Vis | 0.50 ppm 0.09 ppm 0.09 ppm 1.48 ppm 0.65 ppm |
1.69 ppm 0.32 ppm 0.32 ppm 4.96 ppm 2.19 ppm |
[25] |
|
Plasma | HPLC | UV-Vis | - | 1.25 ng/mL 1.75 ng/mL |
[26] |
|
Plasma | HPLC | UV-Vis | 1 ng/mL 1 ng/mL |
2 ng/mL 2 ng/mL |
[27] |
|
Milk | HPLC | DAD | 18 lg/kg | 60 lg/kg | [28] |
|
Water | HPLC | UV-Vis | 0.1 ng/mL | 0.5 ng/mL | [30] |
|
Milk formula | HPLC | UV-Vis | 0.01 µg/mL | 0.033 µg/mL | [31] |
|
Urine | HPLC | MS/MS | 0.015 ng/mL 0.015 ng/mL 0.015 ng/mL 0.015 ng/mL |
0.025 ng/mL 0.025 ng/mL 0.020 ng/mL 0.030 ng/mL |
[37] |
|
Urine | HPLC | Fluorescence | 0.01 µg/mL 0.02 µg/mL 0.1 µg/mL 0.001 µg/mL 0.05 µg/mL |
1 µg/mL 0.05 µg/mL 0.3 µg/mL 0.003 µg/mL 0.25 µg/mL |
[43] |
|
Plasma | HPLC | UV-Vis | - | 75 ng/mL 75 ng/mL 75 ng/mL 75 ng/mL 5 ng/mL 6 ng/mL 5 ng/mL 75 ng/mL |
[44] |
|
Urine | UHPLC | MS/MS | 0.0125 ng/mL | 0.1 ng/mL | [45] |
|
Plasma | UPLC | MS/MS | 0.04 µg/mL 01 µg/mL 0.02 µg/mL |
0.15 ug/mL 0.32 ug/mL 0.06 ug/mL |
[46] |
|
Plasma | HPLC | MS/MS | - | 50.2 ng/mL 1.25 ng/mL |
[47] |
|
Wastewater | UPLC | MS/MS | 0.02 ng/mL | 0.05 ng/mL | [48] |
|
Saliva | UPLC | DAD | 3 ng/mL | 5 ng/mL | [49] |
|
Wastewater | CE | UV-Vis | 3 µg/mL 3 µg/mL 3 µg/mL 3 µg/mL |
5 µg/mL 5 µg/mL 5 µg/mL 5 µg/mL |
[50] |
|
Chicken feces | HPLC | DAD | 0.14 mg/mL 0.14 mg/mL |
0.45 mg/mL 0.43 mg/mL |
[51] |
|
Urine | CE | MS/MS | 1 ng/mL 1 ng/mL 0.60 ng/mL |
5 ng/mL 8 ng/mL 2 ng/mL |
[52] |
|
Water | CE | DAD | 0.016 µg/mL 0.040 µg/mL 0.097 µg/mL 0.037 µg/mL 0.037 µg/mL |
0.05 µg/mL 0.14 µg/mL 0.33 µg/mL 0.13 µg/mL 0.13 µg/mL |
[53] |
|
Tea infusions | CE | Conductivity | 0.80 ng/mL 0.56 ng/mL 1.56 ng/mL 0.54 ng/mL |
2.68 ng/mL 1.87 ng/mL 5.19 ng/mL 1.82 ng/mL |
[54] |
|
Milk | CE | UV-Vis | 19.93 ng/mL 23.83 ng/mL 18.60 ng/mL |
59.79 ng/mL 71.49 ng/mL 55.8 ng/mL |
[55] |
|
Milk | CE | UV-Vis | 0.16 µg/mL 0.04 µg/mL 0.03 µg/mL 0.10 µg/mL 0.07 µg/mL 0.03 µg/mL 0.20 µg/mL 0.07 µg/mL |
0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL 0.3 µg/mL |
[56] |
|
Plasma | GC | MS/MS | 0.5 ng/mL | 1 ng/mL | [57] |
|
Foods | CE | UV-Vis | 0.087 ng/mL | 0.29 ng/mL | [58] |
