
Video Upload Options
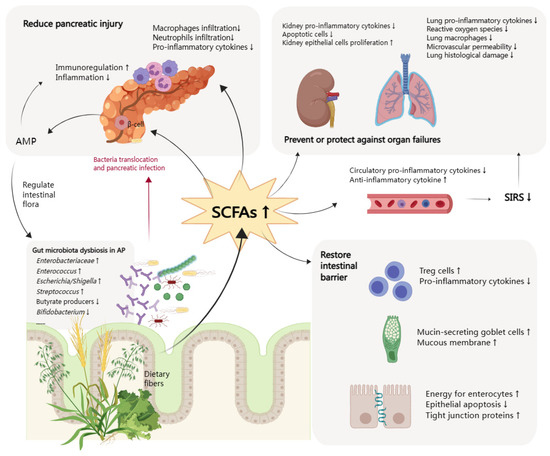
Acute pancreatitis (AP) is a digestive emergency and can develop into a systematic illness. The role of the gut in the progression and deterioration of AP has drawn much attention from researchers, and areas of interest include dysbiosis of the intestinal flora, weakened intestinal barrier function, and bacterial and endotoxin translocation. Short-chain fatty acids (SCFAs), as one of the metabolites of gut microbiota, have been proven to be depleted in AP patients. SCFAs help restore gut homeostasis by rebuilding gut flora, stabilizing the intestinal epithelial barrier, and regulating inflammation. SCFAs can also suppress systematic inflammatory responses, improve the injured pancreas, and prevent and protect other organ dysfunctions. Based on multiple beneficial effects, increasing SCFAs is an essential idea of gut protective treatment in AP. Specific strategies include the direct use of butyrate or indirect supplementation through fiber, pre/pro/synbiotics, or fecal microbiota transplantation as a promising adjective therapy to enteral nutrition.
1. Introduction
2. Function of SCFAs in AP
2.1. Mitigation of Intestinal Injury
2.2. Reduction of Pancreas Injury
2.3. Prevention and Protection of Other Organ Dysfunctions
References
- Banks, P.A.; Freeman, M.L. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006, 101, 2379–2400.
- Leaphart, C.L.; Tepas, J.J., III. The gut is a motor of organ system dysfunction. Surgery 2007, 141, 563–569.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Sakata, T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: A possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br. J. Nutr. 1987, 58, 95–103.
- Pouteau, E.; Nguyen, P.; Ballèvre, O.; Krempf, M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc. Nutr. Soc. 2003, 62, 87–93.
- Reilly, K.J.; Rombeau, J.L. Metabolism and potential clinical applications of short-chain fatty acids. Clin. Nutr. 1993, 12, S97–S105.
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Møller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137.
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135.
- Willemsen, L.E.; Koetsier, M.A.; van Deventer, S.J.; van Tol, E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685.
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356.
- Li, X.Y.; He, C.; Zhu, Y.; Lu, N.H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193.
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 53–64.
- Al-Omran, M.; Albalawi, Z.H.; Tashkandi, M.F.; Al-Ansary, L.A. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst. Rev. 2010, 2010, Cd002837.
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390.
- Wu, L.M.; Sankaran, S.J.; Plank, L.D.; Windsor, J.A.; Petrov, M.S. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br. J. Surg. 2014, 101, 1644–1656.
- Ding, L.; Chen, H.Y.; Wang, J.Y.; Xiong, H.F.; He, W.H.; Xia, L.; Lu, N.H.; Zhu, Y. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J. Gastroenterol. 2020, 26, 514–523.
- Gao, Y.; Davis, B.; Zhu, W.; Zheng, N.; Meng, D.; Walker, W.A. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G521–G530.
- Xiong, Y.; Ji, L.; Zhao, Y.; Liu, A.; Wu, D.; Qian, J. Sodium Butyrate Attenuates Taurocholate-Induced Acute Pancreatitis by Maintaining Colonic Barrier and Regulating Gut Microorganisms in Mice. Front. Physiol. 2022, 13, 813735.
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243.
- Xiao, S.; Jing, S.; Jiakui, S.; Lei, Z.; Ying, L.; Han, L.; Xinwei, M.; Weiqin, L. Butyrate Ameliorates Intestinal Epithelial Barrier Injury Via Enhancing Foxp3+ Regulatory T-Cell Function in Severe Acute Pancreatitis Model. Turk. J. Gastroenterol. 2022, 33, 710–719.
- Harrison, O.J.; Powrie, F.M. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb. Perspect. Biol. 2013, 5.
- Barnes, M.J.; Powrie, F. Regulatory T cells reinforce intestinal homeostasis. Immunity 2009, 31, 401–411.
- Ke, L.; Ni, H.B.; Sun, J.K.; Tong, Z.H.; Li, W.Q.; Li, N.; Li, J.S. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J. Surg. 2012, 36, 171–178.
- Ke, L.; Tong, Z.H.; Ni, H.B.; Ding, W.W.; Sun, J.K.; Li, W.Q.; Li, N.; Li, J.S. The effect of intra-abdominal hypertension incorporating severe acute pancreatitis in a porcine model. PLoS ONE 2012, 7, e33125.
- Gong, G.; Wang, P.; Ding, W.; Zhao, Y.; Li, J. The role of oxygen-free radical in the apoptosis of enterocytes and bacterial translocation in abdominal compartment syndrome. Free Radic. Res. 2009, 43, 470–477.
- Zhao, H.B.; Jia, L.; Yan, Q.Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and Butyrate on Intestinal Barrier Functions: Study of a Rat Model of Severe Acute Pancreatitis With Intra-Abdominal Hypertension. Front. Physiol. 2020, 11, 561061.
- Yan, Q.; Jia, L.; Wen, B.; Wu, Y.; Zeng, Y.; Wang, Q. Clostridium butyricum Protects Against Pancreatic and Intestinal Injury After Severe Acute Pancreatitis via Downregulation of MMP9. Front. Pharmacol. 2022, 13, 919010.
- Kocael, A.; Inal, B.B.; Guntas, G.; Kelten, C.; Inal, H.; Topac, H.I.; Kocael, P.; Simsek, O.; Karaca, G.; Salihoglu, Z.; et al. Evaluation of matrix metalloproteinase, myeloperoxidase, and oxidative damage in mesenteric ischemia-reperfusion injury. Hum. Exp. Toxicol. 2016, 35, 851–860.
- Zhang, H.; Liu, L.; Jiang, C.; Pan, K.; Deng, J.; Wan, C. MMP9 protects against LPS-induced inflammation in osteoblasts. Innate Immun. 2020, 26, 259–269.
- Apostolidou, E.; Paraskeva, E.; Gourgoulianis, K.; Molyvdas, P.A.; Hatzoglou, C. Matrix metalloproteinases 2 and 9 increase permeability of sheep pleura in vitro. BMC Physiol. 2012, 12, 2.
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity 2015, 43, 304–317.
- Pan, X.; Fang, X.; Wang, F.; Li, H.; Niu, W.; Liang, W.; Wu, C.; Li, J.; Tu, X.; Pan, L.L.; et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br. J. Pharmacol. 2019, 176, 4446–4461.
- Lei, Y.; Tang, L.; Liu, S.; Hu, S.; Wu, L.; Liu, Y.; Yang, M.; Huang, S.; Tang, X.; Tang, T.; et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 2021, 9, 115.
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111.
- Yamada, T.; Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Yamakawa, K.; Hamasaki, T.; Nakahori, Y.; Ohnishi, M.; Kuwagata, Y.; et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J. Parenter. Enteral Nutr. 2015, 39, 569–577.
- Zhang, T.; Xia, M.; Zhan, Q.; Zhou, Q.; Lu, G.; An, F. Sodium Butyrate Reduces Organ Injuries in Mice with Severe Acute Pancreatitis Through Inhibiting HMGB1 Expression. Dig. Dis. Sci. 2015, 60, 1991–1999.
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S. The Inflammation Induced by Lipopolysaccharide can be Mitigated by Short-chain Fatty Acid, Butyrate, through Upregulation of IL-10 in Septic Shock. Scand. J. Immunol. 2017, 85, 258–263.
- Jacobs, M.L.; Daggett, W.M.; Civette, J.M.; Vasu, M.A.; Lawson, D.W.; Warshaw, A.L.; Nardi, G.L.; Bartlett, M.K. Acute pancreatitis: Analysis of factors influencing survival. Ann. Surg. 1977, 185, 43–51.
- Shields, C.J.; Winter, D.C.; Redmond, H.P. Lung injury in acute pancreatitis: Mechanisms, prevention, and therapy. Curr. Opin. Crit. Care 2002, 8, 158–163.
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321.
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L65–L78.
- Tian, X.; Hellman, J.; Horswill, A.R.; Crosby, H.A.; Francis, K.P.; Prakash, A. Elevated Gut Microbiome-Derived Propionate Levels Are Associated With Reduced Sterile Lung Inflammation and Bacterial Immunity in Mice. Front. Microbiol. 2019, 10, 159.
- Prakash, A.; Sundar, S.V.; Zhu, Y.G.; Tran, A.; Lee, J.W.; Lowell, C.; Hellman, J. Lung Ischemia-Reperfusion is a Sterile Inflammatory Process Influenced by Commensal Microbiota in Mice. Shock 2015, 44, 272–279.
- Li, N.; Liu, X.X.; Hong, M.; Huang, X.Z.; Chen, H.; Xu, J.H.; Wang, C.; Zhang, Y.X.; Zhong, J.X.; Nie, H.; et al. Sodium butyrate alleviates LPS-induced acute lung injury in mice via inhibiting HMGB1 release. Int. Immunopharmacol. 2018, 56, 242–248.
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682.
- Xu, M.; Wang, C.; Li, N.; Wang, J.; Zhang, Y.; Deng, X. Intraperitoneal Injection of Acetate Protects Mice Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury Through Its Anti-Inflammatory and Anti-Oxidative Ability. Med. Sci. Monit. 2019, 25, 2278–2288.
- Karoor, V.; Strassheim, D.; Sullivan, T.; Verin, A.; Umapathy, N.S.; Dempsey, E.C.; Frank, D.N.; Stenmark, K.R.; Gerasimovskaya, E. The Short-Chain Fatty Acid Butyrate Attenuates Pulmonary Vascular Remodeling and Inflammation in Hypoxia-Induced Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 9916.
- Hu, X.; Han, Z.; Zhou, R.; Su, W.; Gong, L.; Yang, Z.; Song, X.; Zhang, S.; Shu, H.; Wu, D. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Front. Cell. Infect. Microbiol. 2023, 13, 1127369.
- Devani, K.; Charilaou, P.; Radadiya, D.; Brahmbhatt, B.; Young, M.; Reddy, C. Acute pancreatitis: Trends in outcomes and the role of acute kidney injury in mortality- A propensity-matched analysis. Pancreatology 2018, 18, 870–877.
- Nassar, T.I.; Qunibi, W.Y. AKI Associated with Acute Pancreatitis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1106–1115.
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888.
- Liu, Y.; Li, Y.J.; Loh, Y.W.; Singer, J.; Zhu, W.; Macia, L.; Mackay, C.R.; Wang, W.; Chadban, S.J.; Wu, H. Fiber Derived Microbial Metabolites Prevent Acute Kidney Injury Through G-Protein Coupled Receptors and HDAC Inhibition. Front. Cell Dev. Biol. 2021, 9, 648639.
- Besselink, M.G.; van Santvoort, H.C.; Boermeester, M.A.; Nieuwenhuijs, V.B.; van Goor, H.; Dejong, C.H.; Schaapherder, A.F.; Gooszen, H.G. Timing and impact of infections in acute pancreatitis. Br. J. Surg. 2009, 96, 267–273.
- Marik, P.E.; Zaloga, G.P. Early enteral nutrition in acutely ill patients: A systematic review. Crit. Care Med. 2001, 29, 2264–2270.




