In AP patients, there are changes in intestinal flora diversity and composition. The increase of pathogenic bacteria and the reduction of probiotics have been detected, as well as decreased levels of short-chain fatty acids (SCFAs). SCFAs, mainly including acetate, propionate, and butyrate, are produced by microbial fermentation of undigested dietary carbohydrates in the intestine. There is a biological gradient for SCFAs from the gut lumen to the periphery [
3]. As one of the intestinal flora’s metabolites, SCFAs directly provide energy for intestinal mucosal epithelial cells. The remaining part can be absorbed into the bloodstream to provide energy to other cells in the body [
4,
5]. SCFAs participate in glucose and lipid metabolism as substrates after transporting to hepatocytes and adipocytes and regulate appetite by interplaying with neurons [
6,
7]. SCFAs are also important signaling molecules involved in stabilizing the intestinal barrier and promoting intestinal immunity. Specifically, SCFAs protect the intestinal barrier by regulating the expression and distribution of tight junction proteins and promoting the secretion of mucin on the intestinal surface [
8,
9]. Moreover, SCFAs suppress the production of pro-inflammatory cytokines and promote immune cell recruitment, thus being regarded as a potential bioactive molecule for treating intestinal diseases [
10,
11]. There are two major functional pathways of SCFAs: the inhibition of histone deacetylase (HDAC) to exert epigenetic effects and the activation of G protein-coupled receptors (GPRs) to transfer signals [
12]. Microbiota alterations and the decreased production of SCFAs are involved in the increase of intestinal permeability, leading to intestinal bacterial translocation, pancreatic tissue necrosis and infection, and even sepsis and MODS [
13].
2. Function of SCFAs in AP
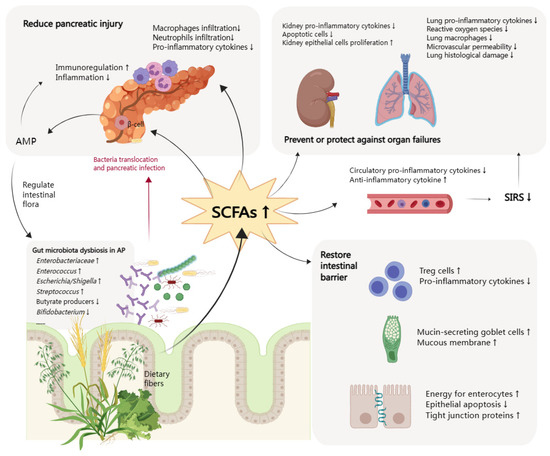
2.1. Mitigation of Intestinal Injury
Gut barrier dysfunction is present in three of five patients with AP, which is associated with poor clinical outcomes [
31]. Considering the site of SCFAs production, most studies on the mechanism of SCFAs focused on intestinal homeostasis [
32]. Intestinal homeostasis is an organic and dynamic balancing state involving the gut microbiota, the intestinal epithelial barrier, and the mucosal immune barrier. The protective role of SCFAs can also be summarized in these three aspects. SCFAs can rebuild the disordered intestinal flora. After butyrate supplementation, the abundance of SCFA-producing
Alloprevotella and
Muribaculaceae increased [
33,
34]. SCFAs also act directly on the intestinal epithelium to protect the integrity of the intestinal barrier, which can be observed at the histological level. Moreover, SCFAs are a critical carbon source for colonic enterocytes [
35]. SAP rats in the butyrate treatment group showed mitigated mucosa lesions and decreased epithelial apoptosis. Butyrate protected the intestinal barrier by upregulating tight junction proteins, such as zonula occludens-1 (ZO-1), claudin, and occludin [
33,
34]. In addition to the epithelial barrier repairment, butyrate also well-restored mucin-secreting goblet cells, thus protecting the damaged mucous membrane [
33]. For the immunity barrier, pre-treatment with sodium butyrate ameliorated intestinal inflammation and injury by reducing intestinal pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6. Butyrate also increased the expression of Foxp3 at both mRNA and protein levels, detected in immunofluorescence staining and flow cytometry analysis. These results supported the elevated percentage of regulatory T cells (CD4+, CD25+, Foxp3+), which could maintain intestinal homeostasis by preventing inappropriate innate and adaptive immune responses [
36,
37,
38].
SAP patients can develop intraabdominal hypertension (IAH), which can progress to abdominal compartment syndrome (ACS) with a high mortality rate of 66.7%. These severe abdominal complications may correlate with pancreatic necrosis and intra-abdominal infection, likely resulting from bacterial translocation [
39,
40,
41].
Clostridium butyricum is an anaerobic bacterium that can ferment dietary fibers to produce SCFAs. SAP + IAH rats who received oral
C. butyricum or butyrate had reduced pathological severity scores of intestinal injury and plasma levels of inflammatory markers. Compared with the nontreated group, the expression of ZO-1, claudin-1, and occludin increased, and claudin-2, matrix metallopeptidases 9 (MMP9), and TNF-a lowered in the treatment group, indicating repairment of the intestinal mucosal barrier. The treatment also rebuilt the intestinal flora, significantly increasing richness and diversity, growing probiotics (
Lactobacillus,
Coprococcus, and
Allobaculum), and decreasing pathogenic species (
Bacteroides,
Escherichia,
Helicobacter, and
Desulfovibrio) [
42]. These multiple reversed pathological responses suggested butyrate supplementation as a promising therapeutic strategy to restore intestinal function. Another study further confirmed the protective effect of
C. butyricum or butyrate via downregulating MMP9 expression [
43]. MMP9 was upregulated in intestinal tissues of the SAP model according to existing studies [
44,
45], and is one of the members of MMPs that can degrade and remodel extracellular matrix. MMPs are also involved in the inflammation process and intestinal barrier injury. For example, MMPs can increase endothelial cell permeability by disrupting tight junction proteins [
46]. Additionally, Kocael et al. reported that MMP9 overexpression also facilitated the loss of intestinal villous in the mesenteric ischemia-reperfusion injury model [
44]. Therefore, MMP9 is a vital molecule mediating intestinal injury and a potential target of SCFA supplementation.
2.2. Reduction of Pancreas Injury
There has been evidence for the direct interaction between the pancreas and SCFAs and the existence of the gut–pancreatic axis [
47]. Cathelicidin-related antimicrobial peptide (CRAMP) production by insulin-secreting beta-cells is controlled by SCFAs produced by the gut microbiota. However, local functions of SCFAs in the pancreatic tissue of AP are limitedly studied. In the AP mice model, butyrate mitigated the severity of AP in multiple ways, reflected in both the pancreas and the gut. A study provided new insights into tissue-specific mechanisms of butyrate. Pre-treatment with sodium butyrate decreased the infiltration of macrophages and neutrophils in the pancreas and reduced levels of intestinal pro-inflammation cytokines. Sodium butyrate acted as an HDAC1 inhibitor in the pancreas or as a GPR109A agonist in the colon to suppress the activation of NLRP3 inflammasome [
48]. Lei et al. also reported that, in the heparanase-exacerbated AP model, the supplementation of Parabacteroides or sodium acetate could reduce neutrophils in blood and infiltration in the pancreas [
49]. Similarly, in another study, sodium butyrate supplementation significantly reduced the proportion of neutrophils, macrophages, and M2-type macrophages in the pancreatic tissue from AP mice and inhibited IL-1b, CXCL1, and TNF-a levels [
34].
2.3. Prevention and Protection of Other Organ Dysfunctions
In the early phase of AP, inflammation of the pancreas activates cytokine cascades, which are clinically manifested as SIRS [
50]. Avoidance of SIRS or timely termination of SIRS is the key to early control of AP. Fecal concentrations of butyrate, propionate, and acetate in patients with severe SIRS on admission decreased significantly compared with those in healthy volunteers. They remained low throughout the six weeks of intensive care unit (ICU) stay. In patients with gastrointestinal complications, including enteritis and dysmotility, the level of SCFAs was even lower [
51]. Zhang et al. found that sodium butyrate treatment could inhibit the nuclear factor-κB (NF-κB) signaling pathway and lower the expression of High-mobility group box-1 (HMGB1), which is a late cytokine mediator stimulating the release of pro-inflammatory cytokines. The SAP model had reduced pathological lesions; reduced serum levels of HMGB1, TNF-a, and IL-6; as well as diminished HMGB1 mRNA levels and NF-κB activity [
52]. Another study that used the SAP model also evaluated the plasma levels of several markers. The administration of
Clostridium butyricum or butyrate reduced pro-inflammatory cytokines, including TNF-a, IL-6, IL-1β, and IL-12 [
42]. What is more, SCFAs mitigated the inflammation in the lipopolysaccharide (LPS)-induced septic shock model by upregulating the anti-inflammatory cytokine IL-10 [
53].
Patients with persistent SIRS are at risk for one or more organ failures, the leading cause of early death. Three organ systems should be assessed to define organ failure, including respiratory, cardiovascular, and renal systems. Organ failure may be transient with remission within 48 h in MSAP or persistent for more than 48 h in SAP [
50]. SCFAs prevent or protect against organ failures by restoring the intestinal barrier and suppressing systematic inflammatory responses. Some changes mediated by SCFAs in specifically targeted organs, such as the lung and kidney, are also observed.
One-third of patients presenting with severe AP develop acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). The primary manifestation is hypoxemia, which can even develop into acute respiratory distress syndrome (ARDS). The lung injury is characterized by increased pulmonary microvasculature permeability and subsequent protein-rich exudate leakage into the alveolar spaces, forming the hyaline membrane [
54,
55]. The concept of the gut–lung axis has been proposed based on the bidirectional crosstalk between these two organs [
56]. SCFAs provide one of these crosstalk pathways. Human lung tissue contained variable acetate and propionate levels, likely originating from the gut and transiting to the lung. SCFA receptors, namely free fatty acid receptor (FFAR) 2 and FFAR3, were expressed in vitro in alveolar macrophages (AM) and alveolar type 2 epithelial (AT2) cells, and exposure to LPS regulated this expression. This finding supported the direct effects of SCFAs on the lung [
57]. Specifically, gut microbiota-produced LPS and SCFAs could strongly influence the course of lung injury and infections [
58,
59]. SCFAs significantly protected animals from LPS-induced ALI, as evidenced by suppressed HMGB1 release and NF-κB activation, decreased production of pro-inflammatory cytokines and reactive oxygen species, declined immune cell counting, and alleviated LPS-induced microvascular permeability and lung histological damage [
60,
61,
62]. In the hypoxic model, butyrate treatment decreased the accumulation of alveolar and interstitial lung macrophages, prevented hypoxia-induced pulmonary vascular edema and vascular leakage, and upregulated the expression of tight junctions in lung microvascular endothelial cells [
63]. Tian et al. similarly found that enrichment of propionate-producing gut bacteria (especially
Lachnospiraceae) was related to reduced lung inflammation following lung ischemia-reperfusion injury in vivo [
58]. Compared with AP patients without ARDS, AP with ARDS had higher abundances of the Proteobacteria phylum, the
Enterobacteriaceae family,
Escherichia-Shigella, and the
Klebsiella pneumoniae genus but lower abundances of the
Bifidobacterium genus [
64]. Thus, gut microbiota and SCFAs may play essential roles in pancreatitis-associated lung injury through the above mechanisms, although no studies used the AP model.
Acute kidney injury (AKI) is another frequent complication of SAP. A comprehensive, retrospective, observational study reported an overall AKI prevalence of 7.9% among hospitalized patients with AP [
65]. The pathogenesis may include increased vascular permeability, hypovolemia, inflammation, vasoconstriction, intravascular coagulation, and direct nephrotoxic effects [
66]. Predictors for a higher likelihood of AKI include higher age, exhibiting biological male sex, a more significant number of co-morbidities, and electrolyte imbalance [
65]. Andrade-Oliveira et al. observed that therapy with the three main SCFAs (acetate, propionate, and butyrate) improved renal dysfunction. In specific, SCFAs treatment reduced pro-inflammatory cytokines and chemokines in kidney tissue and serum, with low levels of toll-like receptor 4 (TLR4) mRNA and lesser activation of the NF-κB pathway. SCFA treatment also diminished apoptotic cells in kidney tissue, but increased the proliferation of kidney epithelial cells, thus promoting the restoration of injured tissue. Mice treated with acetate-producing bacteria also achieved better outcomes after AKI, having increased acetate levels in feces and plasma, low serum levels of creatinine and urea, and low serum levels of cytokines and chemokines [
67]. Another study showed that a high-fiber diet had similar protective effects for AKI [
68]. Zhang et al. evaluated other organs in the SAP model after sodium butyrate treatment and found alleviated liver and renal tissue histological injuries and improved hepatic and renal function reflected in decreased alanine aminotransferase and creatine levels [
52].
The late phase of AP is characterized by the persistence of systematic signs of inflammation or local complications, with an increased risk of infection. Up to 20% of AP patients develop extra-pancreatic infections, such as bloodstream infections, pneumonia, and urinary tract infections [
69]. In a meta-analysis of studies performed in the ICU, there was a significantly lower risk of infection in the patients who received early enteral nutrition. Infectious complications occurred in 19% of the early nutritional group compared to 41% in the delayed group [
70]. Considering that SCFAs can protect the intestinal barrier and prevent bacteria translocation, SCFAs are a good choice to reduce the incidence of systemic infectious complications of AP as a supplement to early enteral nutrition. The above-mentioned possible mechanisms of SCFAs in AP are summarized in
Figure 1.
Figure 1. Function of short chain fatty acids in acute pancreatitis. SCFAs, short chain fatty acids; AMP, antimicrobial peptide; SIRS, systemic inflammatory response syndrome; AP, acute pancreatitis; Treg cells, regulatory T cells. The arrows (↑ and ↓) indicate the alternation of gut microbiota in AP or histopathological changes after increasing SCFAs.