
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simonetta Genovesi | -- | 2721 | 2023-06-30 13:58:55 | | | |
| 2 | Rita Xu | Meta information modification | 2721 | 2023-07-03 03:57:43 | | |
Video Upload Options
Lipoprotein(a) [Lp(a)] is made up of apoprotein(a) [apo(a)] and LDL-like particle. The proportion of Lp(a) in each individual is genetically determined and is only minimally modifiable by environment or diet. Lp(a) has important pro-atherosclerotic and pro-inflammatory effects. For these reasons, high Lp(a) values are an important independent risk factor for cardiovascular disease and calcific aortic valve stenosis. Numerous studies have been performed in adults about the pathophysiology and epidemiology of Lp(a), much less information is available regarding Lp(a) in children and adolescents. Gaining information on these points is particularly important for deciding whether Lp(a) assay may be useful for defining the cardiovascular risk in children, in order to plan a prevention program early.
1. Introduction
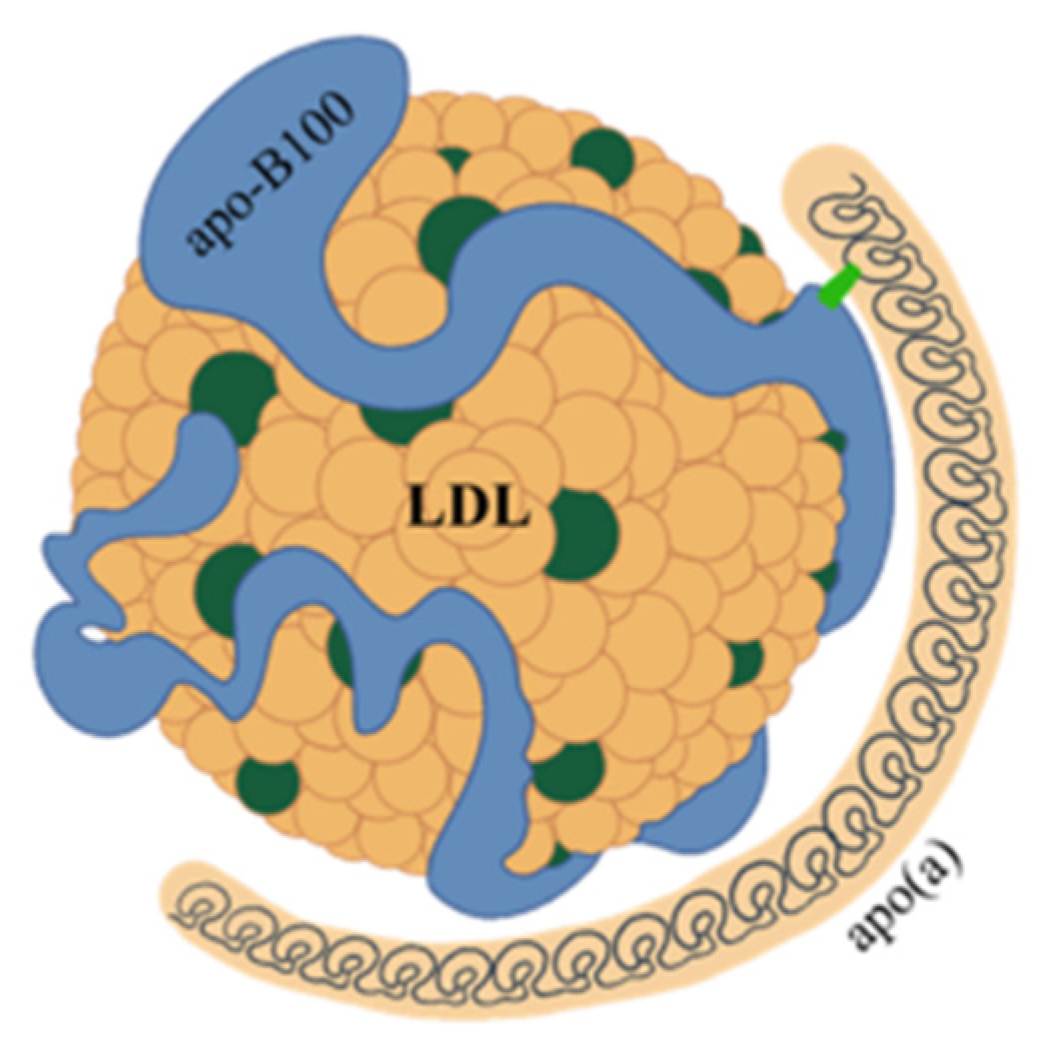
2. Structure and Features of Lp(a)

3. Lp(a) and Cardiovascular Risk
4. Lp(a) in Adults
References
- Berg, K. A new serum type system in man—The lp system. Acta Pathol. Microbiol. Scand. 1963, 59, 369–382.
- McLean, J.W.; Tomlinson, J.E.; Kuang, W.J.; Eaton, D.L.; Chen, E.Y.; Fless, G.M.; Scanu, A.M.; Lawn, R.M. CDNA Sequence of Human Apolipoprotein(a) Is Homologous to Plasminogen. Nature 1987, 330, 132–137.
- Erqou, S.; Thompson, A.; Di Angelantonio, E.; Saleheen, D.; Kaptoge, S.; Marcovina, S.; Danesh, J. Apolipoprotein(a) Isoforms and the Risk of Vascular Disease: Systematic Review of 40 Studies Involving 58,000 Participants. J. Am. Coll. Cardiol. 2010, 55, 2160–2167.
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009, 301, 2331–2339.
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic Associations with Valvular Calcification and Aortic Stenosis. N. Engl. J. Med. 2013, 368, 503–512.
- Langsted, A.; Nordestgaard, B.G.; Kamstrup, P.R. Elevated Lipoprotein(a) and Risk of Ischemic Stroke. J. Am. Coll. Cardiol. 2019, 74, 54–66.
- Koschinsky, M.L.; Kronenberg, F. The Long Journey of Lipoprotein(a) from Cardiovascular Curiosity to Therapeutic Target. Atherosclerosis 2022, 349, 1–6.
- van der Hoek, Y.Y.; Wittekoek, M.E.; Beisiegel, U.; Kastelein, J.J.; Koschinsky, M.L. The Apolipoprotein(a) Kringle IV Repeats Which Differ from the Major Repeat Kringle Are Present in Variably-Sized Isoforms. Hum. Mol. Genet. 1993, 2, 361–366.
- Santonastaso, A.; Maggi, M.; De Jonge, H.; Scotti, C. High Resolution Structure of Human Apolipoprotein (a) Kringle IV Type 2: Beyond the Lysine Binding Site. J. Lipid Res. 2020, 61, 1687–1696.
- Garner, B.; Merry, A.H.; Royle, L.; Harvey, D.J.; Rudd, P.M.; Thillet, J. Structural Elucidation of the N- and O-Glycans of Human Apolipoprotein(a): Role of o-Glycans in Conferring Protease Resistance. J. Biol. Chem. 2001, 276, 22200–22208.
- Kostner, G.M. Lp(a) Biochemistry, Composition, and Structure. In Lipoprotein(a); Kostner, K., Kostner, G.M., Toth, P.P., Eds.; Contemporary Cardiology; Springer International Publishing: Cham, Switzerland, 2023; pp. 39–54. ISBN 978-3-031-24575-6.
- Zheng, K.H.; Tsimikas, S.; Pawade, T.; Kroon, J.; Jenkins, W.S.A.; Doris, M.K.; White, A.C.; Timmers, N.K.L.M.; Hjortnaes, J.; Rogers, M.A.; et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2019, 73, 2150–2162.
- Chemello, K.; Chan, D.C.; Lambert, G.; Watts, G.F. Recent Advances in Demystifying the Metabolism of Lipoprotein(a). Atherosclerosis 2022, 349, 82–91.
- Koschinsky, M.L.; Marcovina, S.M. Structure-Function Relationships in Apolipoprotein(a): Insights into Lipoprotein(a) Assembly and Pathogenicity. Curr. Opin. Lipidol. 2004, 15, 167–174.
- Becker, L.; Nesheim, M.E.; Koschinsky, M.L. Catalysis of Covalent Lp(a) Assembly: Evidence for an Extracellular Enzyme Activity That Enhances Disulfide Bond Formation. Biochemistry 2006, 45, 9919–9928.
- Mooser, V.; Marcovina, S.M.; White, A.L.; Hobbs, H.H. Kringle-Containing Fragments of Apolipoprotein(a) Circulate in Human Plasma and Are Excreted into the Urine. J. Clin. Investig. 1996, 98, 2414–2424.
- Borrelli, M.J.; Youssef, A.; Boffa, M.B.; Koschinsky, M.L. New Frontiers in Lp(a)-Targeted Therapies. Trends Pharmacol. Sci. 2019, 40, 212–225.
- Pagnan, A.; Kostner, G.; Braggion, M.; Ziron, L. Relationship between “sinking Pre-Beta-Lipoprotein” (Lp(a) Lipoprotein) and Age in a Family Kindred. Gerontology 1982, 28, 381–385.
- Reyes-Soffer, G.; Westerterp, M. Beyond Lipoprotein(a) Plasma Measurements: Lipoprotein(a) and Inflammation. Pharmacol. Res. 2021, 169, 105689.
- Kronenberg, F. Causes and Consequences of Lipoprotein(a) Abnormalities in Kidney Disease. Clin. Exp. Nephrol. 2014, 18, 234–237.
- Barbagelata, L.; Masson, W.; Corral, P.; Lavalle-Cobo, A.; Nogueira, J.P.; Rosa Diez, G. Relationship between Lipoprotein(a) Levels, Cardiovascular Outcomes and Death in Patients with Chronic Kidney Disease: A Systematic Review of Prospective Studies. J. Nephrol. 2023.
- Chan, D.C.; Watts, G.F.; Coll, B.; Wasserman, S.M.; Marcovina, S.M.; Barrett, P.H.R. Lipoprotein(a) Particle Production as a Determinant of Plasma Lipoprotein(a) Concentration Across Varying Apolipoprotein(a) Isoform Sizes and Background Cholesterol-Lowering Therapy. J. Am. Heart Assoc. 2019, 8, e011781.
- Boffa, M.B.; Koschinsky, M.L. Understanding the Ins and Outs of Lipoprotein (a) Metabolism. Curr. Opin. Lipidol. 2022, 33, 185–192.
- Coassin, S.; Kronenberg, F. Lipoprotein(a) beyond the Kringle IV Repeat Polymorphism: The Complexity of Genetic Variation in the LPA Gene. Atherosclerosis 2022, 349, 17–35.
- Noureen, A.; Fresser, F.; Utermann, G.; Schmidt, K. Sequence Variation within the KIV-2 Copy Number Polymorphism of the Human LPA Gene in African, Asian, and European Populations. PLoS ONE 2015, 10, e0121582.
- McCormick, S.P.A.; Schneider, W.J. Lipoprotein(a) Catabolism: A Case of Multiple Receptors. Pathology 2019, 51, 155–164.
- Cegla, J.; France, M.; Marcovina, S.M.; Neely, R.D.G. Lp(a): When and How to Measure It. Ann. Clin. Biochem. 2021, 58, 16–21.
- Kronenberg, F. Lipoprotein(a) Measurement Issues: Are We Making a Mountain out of a Molehill? Atherosclerosis 2022, 349, 123–135.
- Kinpara, K.; Okada, H.; Yoneyama, A.; Okubo, M.; Murase, T. Lipoprotein(a)-Cholesterol: A Significant Component of Serum Cholesterol. Clin. Chim. Acta 2011, 412, 1783–1787.
- van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016, 134, 611–624.
- Garg, P.K.; Guan, W.; Karger, A.B.; Steffen, B.T.; Budoff, M.; Tsai, M.Y. Lipoprotein (a) and Risk for Calcification of the Coronary Arteries, Mitral Valve, and Thoracic Aorta: The Multi-Ethnic Study of Atherosclerosis. J. Cardiovasc. Comput. Tomogr. 2021, 15, 154–160.
- Kaiser, Y.; Daghem, M.; Tzolos, E.; Meah, M.N.; Doris, M.K.; Moss, A.J.; Kwiecinski, J.; Kroon, J.; Nurmohamed, N.S.; van der Harst, P.; et al. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J. Am. Coll. Cardiol. 2022, 79, 223–233.
- Leibundgut, G.; Scipione, C.; Yin, H.; Schneider, M.; Boffa, M.B.; Green, S.; Yang, X.; Dennis, E.; Witztum, J.L.; Koschinsky, M.L.; et al. Determinants of Binding of Oxidized Phospholipids on Apolipoprotein (a) and Lipoprotein (a). J. Lipid Res. 2013, 54, 2815–2830.
- Koschinsky, M.L.; Boffa, M.B. Oxidized Phospholipid Modification of Lipoprotein(a): Epidemiology, Biochemistry and Pathophysiology. Atherosclerosis 2022, 349, 92–100.
- Dzobo, K.E.; Kraaijenhof, J.M.; Stroes, E.S.G.; Nurmohamed, N.S.; Kroon, J. Lipoprotein(a): An Underestimated Inflammatory Mastermind. Atherosclerosis 2022, 349, 101–109.
- Boffa, M.B.; Koschinsky, M.L. Lipoprotein (a): Truly a Direct Prothrombotic Factor in Cardiovascular Disease? J. Lipid Res. 2016, 57, 745–757.
- Nordestgaard, B.G.; Langsted, A. Lipoprotein (a) as a Cause of Cardiovascular Disease: Insights from Epidemiology, Genetics, and Biology. J. Lipid Res. 2016, 57, 1953–1975.
- Kaiser, Y.; Singh, S.S.; Zheng, K.H.; Verbeek, R.; Kavousi, M.; Pinto, S.-J.; Vernooij, M.W.; Sijbrands, E.J.G.; Boekholdt, S.M.; de Rijke, Y.B.; et al. Lipoprotein(a) Is Robustly Associated with Aortic Valve Calcium. Heart 2021, 107, 1422–1428.
- Kaltoft, M.; Sigvardsen, P.E.; Afzal, S.; Langsted, A.; Fuchs, A.; Kühl, J.T.; Køber, L.; Kamstrup, P.R.; Kofoed, K.F.; Nordestgaard, B.G. Elevated Lipoprotein(a) in Mitral and Aortic Valve Calcification and Disease: The Copenhagen General Population Study. Atherosclerosis 2022, 349, 166–174.
- Tsimikas, S.; Gordts, P.L.S.M.; Nora, C.; Yeang, C.; Witztum, J.L. Statin Therapy Increases Lipoprotein(a) Levels. Eur. Heart J. 2020, 41, 2275–2284.
- Awad, K.; Mikhailidis, D.P.; Katsiki, N.; Muntner, P.; Banach, M. Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group Effect of Ezetimibe Monotherapy on Plasma Lipoprotein(a) Concentrations in Patients with Primary Hypercholesterolemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Drugs 2018, 78, 453–462.
- Jaeger, B.R.; Richter, Y.; Nagel, D.; Heigl, F.; Vogt, A.; Roeseler, E.; Parhofer, K.; Ramlow, W.; Koch, M.; Utermann, G.; et al. Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 229–239.
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. N. Engl. J. Med. 2011, 365, 2255–2267.
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Lira Pineda, A.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk. Circulation 2019, 139, 1483–1492.
- Tsushima, T.; Tsushima, Y.; Sullivan, C.; Hatipoglu, B. Lipoprotein(a) and Atherosclerotic Cardiovascular Disease, the Impact of Available Lipid-Lowering Medications on Lipoprotein(a): An Update on New Therapies. Endocr. Pract. 2022, S1530-891X(22)00901-6.
- Ibrahim, S.; Stroes, E.S.G. Therapy of Elevated Lipoprotein(a). In Lipoprotein(a); Kostner, K., Kostner, G.M., Toth, P.P., Eds.; Contemporary Cardiology; Springer International Publishing: Cham, Switzerland, 2023; pp. 347–357. ISBN 978-3-031-24575-6.
- Mehta, A.; Jain, V.; Saeed, A.; Saseen, J.J.; Gulati, M.; Ballantyne, C.M.; Virani, S.S. Lipoprotein(a) and Ethnicities. Atherosclerosis 2022, 349, 42–52.
- Patel, A.P.; Wang, M.; Pirruccello, J.P.; Ellinor, P.T.; Ng, K.; Kathiresan, S.; Khera, A.V. Lp(a) (Lipoprotein) Concentrations and Incident Atherosclerotic Cardiovascular Disease: New Insights from a Large National Biobank. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 465–474.
- Virani, S.S.; Brautbar, A.; Davis, B.C.; Nambi, V.; Hoogeveen, R.C.; Sharrett, A.R.; Coresh, J.; Mosley, T.H.; Morrisett, J.D.; Catellier, D.J.; et al. Associations between Lipoprotein(a) Levels and Cardiovascular Outcomes in Black and White Subjects: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2012, 125, 241–249.
- Tsimikas, S.; Clopton, P.; Brilakis, E.S.; Marcovina, S.M.; Khera, A.; Miller, E.R.; de Lemos, J.A.; Witztum, J.L. Relationship of Oxidized Phospholipids on Apolipoprotein B-100 Particles to Race/Ethnicity, Apolipoprotein(a) Isoform Size, and Cardiovascular Risk Factors: Results from the Dallas Heart Study. Circulation 2009, 119, 1711–1719.
- Deo, R.C.; Wilson, J.G.; Xing, C.; Lawson, K.; Kao, W.H.L.; Reich, D.; Tandon, A.; Akylbekova, E.; Patterson, N.; Mosley, T.H.; et al. Single-Nucleotide Polymorphisms in LPA Explain Most of the Ancestry-Specific Variation in Lp(a) Levels in African Americans. PLoS ONE 2011, 6, e14581.
- Paré, G.; Çaku, A.; McQueen, M.; Anand, S.S.; Enas, E.; Clarke, R.; Boffa, M.B.; Koschinsky, M.; Wang, X.; Yusuf, S.; et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation 2019, 139, 1472–1482.
- Varvel, S.; McConnell, J.P.; Tsimikas, S. Prevalence of Elevated Lp(a) Mass Levels and Patient Thresholds in 532 359 Patients in the United States. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2239–2245.
- Trinder, M.; Paruchuri, K.; Haidermota, S.; Bernardo, R.; Zekavat, S.M.; Gilliland, T.; Januzzi, J.; Natarajan, P. Repeat Measures of Lipoprotein(a) Molar Concentration and Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 79, 617–628.
- Jenner, J.L.; Ordovas, J.M.; Lamon-Fava, S.; Schaefer, M.M.; Wilson, P.W.; Castelli, W.P.; Schaefer, E.J. Effects of Age, Sex, and Menopausal Status on Plasma Lipoprotein(a) Levels. The Framingham Offspring Study. Circulation 1993, 87, 1135–1141.
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid Changes during the Menopause Transition in Relation to Age and Weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361.
- Anagnostis, P.; Antza, C.; Trakatelli, C.; Lambrinoudaki, I.; Goulis, D.G.; Kotsis, V. The Effect of Menopause on Lipoprotein (a) Concentrations: A Systematic Review and Meta-Analysis. Maturitas 2023, 167, 39–45.
- Simony, S.B.; Mortensen, M.B.; Langsted, A.; Afzal, S.; Kamstrup, P.R.; Nordestgaard, B.G. Sex Differences of Lipoprotein(a) Levels and Associated Risk of Morbidity and Mortality by Age: The Copenhagen General Population Study. Atherosclerosis 2022, 355, 76–82.




