
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antti Nykaenen | -- | 1720 | 2023-06-29 23:33:10 | | | |
| 2 | Peter Tang | + 1 word(s) | 1721 | 2023-06-30 03:35:14 | | |
Video Upload Options
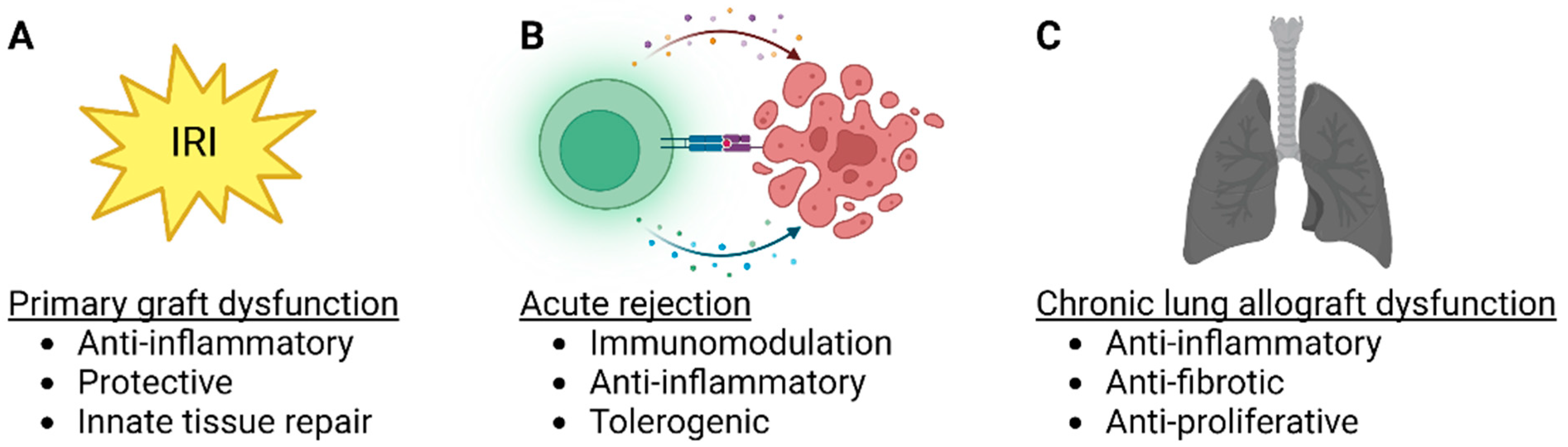
Lung transplantation is often the only viable treatment option for a patient with end-stage lung disease. Lung transplant results have improved substantially over time, but ischemia-reperfusion injury, primary graft dysfunction, acute rejection, and chronic lung allograft dysfunction (CLAD) continue to be significant problems. Mesenchymal stromal cells (MSC) are pluripotent cells that have anti-inflammatory and protective paracrine effects and may be beneficial in solid organ transplantation.
1. Introduction
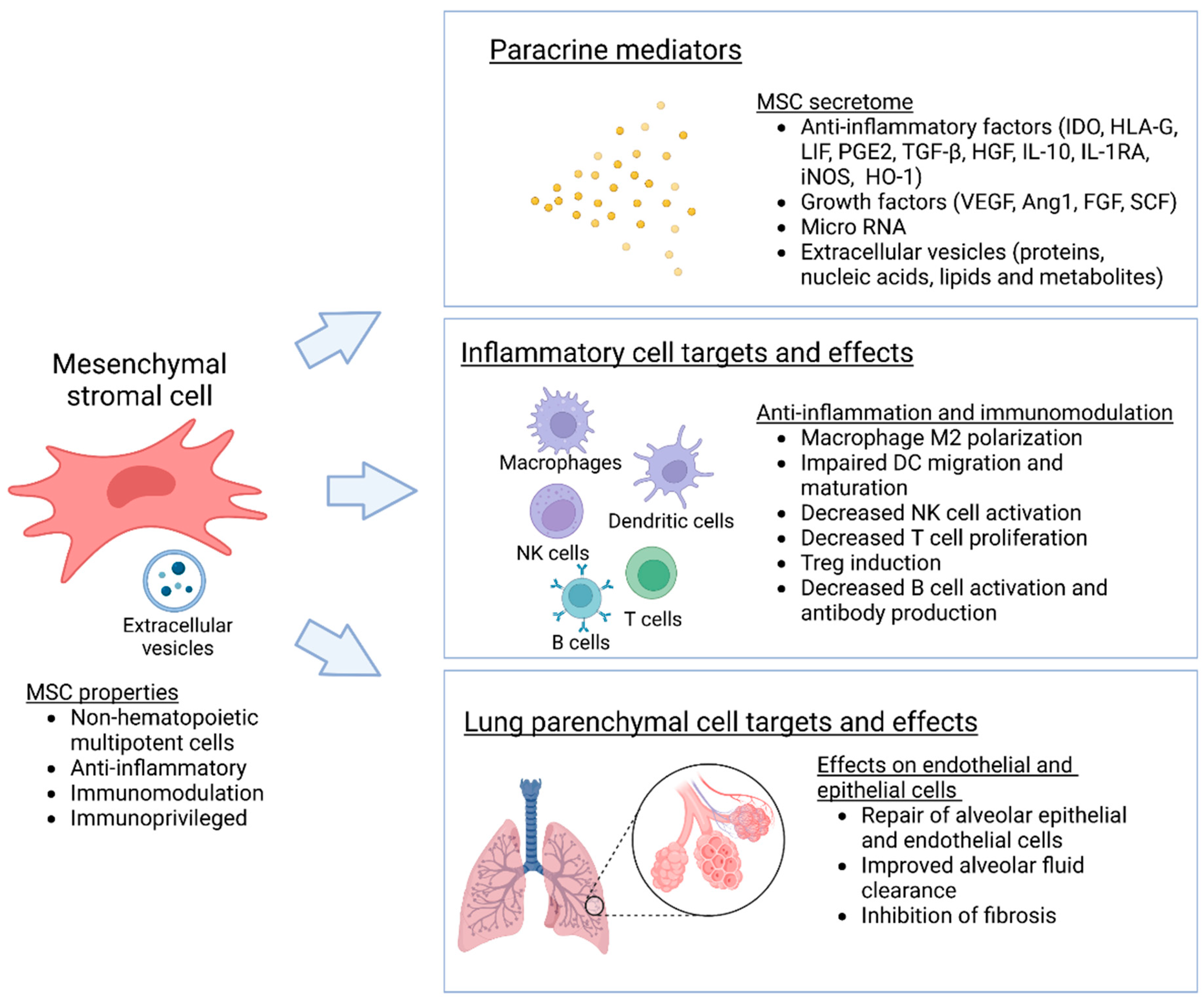
2. Mesenchymal Stromal Cells
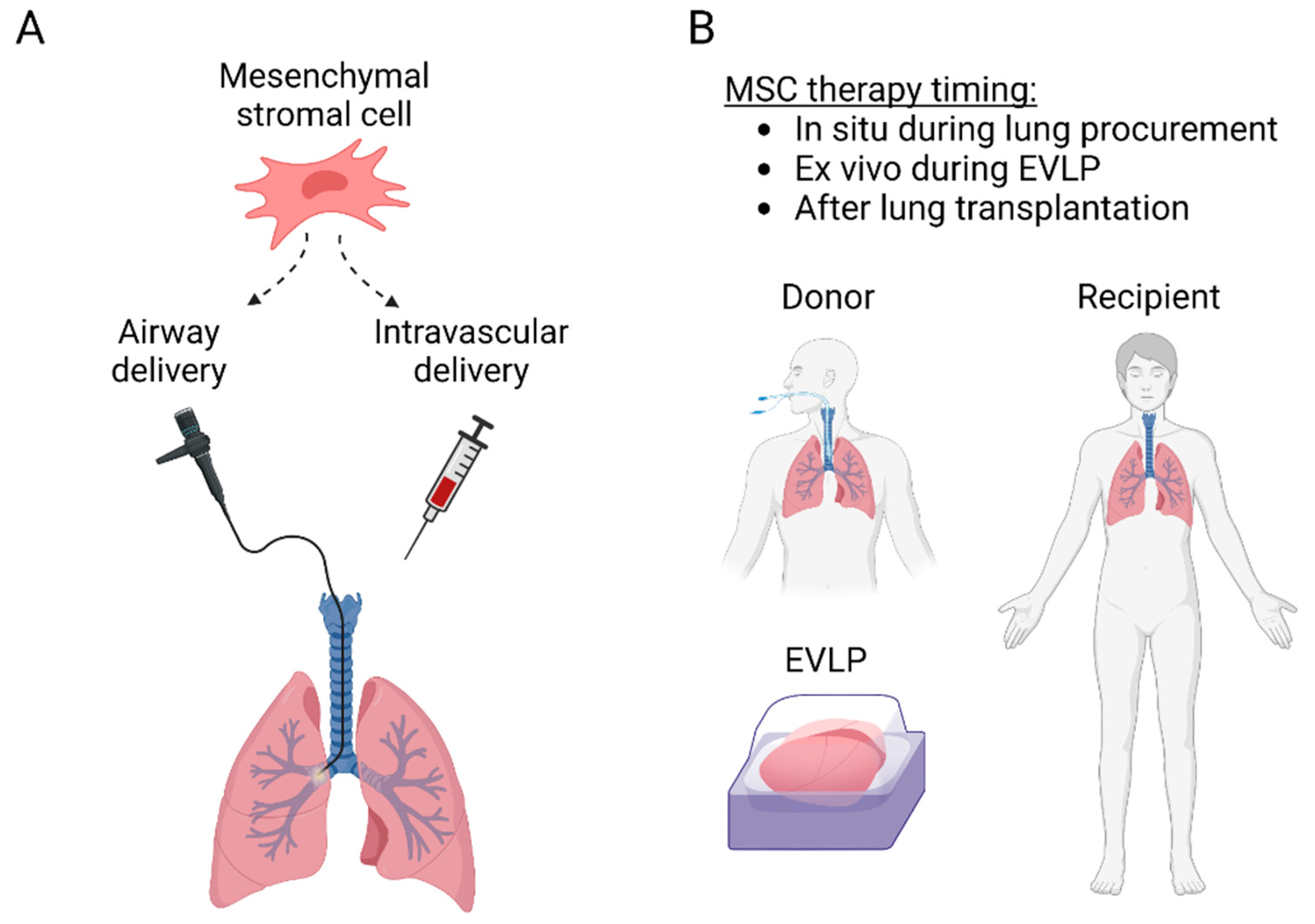
3. Lung Transplant MSC Therapy
|
Target |
Species |
Model |
MSC |
Administration |
Effect |
Ref |
|---|---|---|---|---|---|---|
|
IRI |
Rat |
Lung hilar clamping |
Engineered BM-MSC (MSC-vIL-10) |
Intravenous |
Improved oxygenation, inflammation and permeability |
[22] |
|
IRI |
Pig |
SLTx |
BM-MSC |
Pulmonary artery vs. endobronchial |
Endobronchial MSC delivery improved lung compliance |
[23] |
|
IRI |
Human |
EVLP |
MAPC |
Airways |
Decreased edema and inflammation |
[24] |
|
IRI |
Human |
EVLP |
BM-MSC |
Intravascular |
Restored alveolar fluid clearance |
[25] |
|
IRI |
Mouse |
SLTx |
BM-MSC |
Recipient intravenous |
Decreased IRI, MSC homing preferentially into the lung transplant |
[26] |
|
IRI |
Pig |
EVLP |
UC-MSC |
Airway vs. intravascular, 3 different doses |
Intravascular delivery improved MSC lung retention, optimal dose 150 × 106 MSC decreased IL-8 and increased VEGF |
[27] |
|
IRI |
Mouse |
SLTx |
BM-MSC |
Ex vivo pulmonary artery |
Decreased IRI |
[28] |
|
IRI |
Pig |
SLTx |
BM-MSC |
Pulmonary artery vs. endobronchial |
No short-term differences detected |
[29] |
|
IRI |
Mouse |
Lung hilar clamping and EVLP |
Human UC-MSC vs. MSC-EVs |
Intravascular |
MSCs and MSC-EVs attenuate IRI |
[30] |
|
IRI |
Human |
EVLP |
MAPC |
Airways |
Decreased BAL neutrophilia, TNF-α, IL-1β and IFN-γ |
[31] |
|
IRI |
Pig |
SLTx |
BM-MSC |
Intravenous or intrabronchial |
Heterogenous localization, in alveoli after endobronchial and in blood vessels after intravascular administration |
[32] |
|
IRI |
Rat |
SLTx |
BM-MSC |
Intravenous |
Protection against IRI |
[33] |
|
IRI |
Pig |
EVLP and SLTx |
UC-MSC |
Intravascular |
Decreased IRI during EVLP and after TX |
[34] |
|
IRI |
Rat |
EVLP |
UC-MSC |
Intravascular |
Improved inflammation and IRI |
[35] |
|
IRI |
Rat |
EVLP |
BM-MSC-EVs |
Intravascular |
Multiple influences on pulmonary energetics, tissue integrity and gene expression |
[36] |
|
IRI |
Human |
EVLP |
Engineered UC-MSC (MSCIL−10) |
Intravascular |
Safe and feasible, results in rapid IL-10 elevation |
[37] |
|
IRI |
Rat |
SLTx |
Donor vs. recipient adipose tissue MSC |
Intravenous |
MSCs, regardless of their origin, exert similar immunosuppressive effects |
[38] |
|
IRI/ ARDS |
Human |
EVLP/endotoxin |
BM-MSC |
Airways |
Restored alveolar fluid clearance |
[39] |
|
IRI/ ARDS |
Human |
EVLP/e.coli pneumonia |
BM-MSC |
Airways |
Restored alveolar fluid clearance, reduced inflammation and increased antimicrobial activity |
[40] |
|
Acute rejection |
Rat |
SLTx |
BM-MSC |
1 vs. 2 recipient intravenous doses |
Protection from acute rejection, best result with 2 recipient doses |
[41] |
|
Acute rejection/ CLAD |
Mouse |
Ortotopic tracheal Tx |
iPSC-MSC |
Intravascular |
Induces immune tolerance and supports long-term graft survival |
[42] |
|
CLAD |
Mouse |
Heterotopic tracheal Tx |
MSC (various sources) |
Intravenous |
Prevents airway occlusion |
[43] |
|
CLAD |
Mouse |
Ortotopic tracheal Tx |
BM-MSC |
Intravenous |
Prevents airway occlusion through macrophage cytokines |
[44] |
|
CLAD |
Mouse |
Heterotopic tracheal Tx |
BM-MSC |
Local vs. systemic vs. combination |
Prevents airway occlusion through modulation of immune response, best effect with combination treatment |
[45] |
|
CLAD |
Human |
Clinical Tx |
BM-MSC |
Intravenous twice weekly for 2 weeks |
Safe and feasible in patients with advanced CLAD |
[13] |
|
CLAD |
Human |
Clinical Tx |
BM-MSC |
Intravenous |
Safe and feasible in patients with moderate CLAD |
[12] |
|
CLAD |
Human |
Clinical Tx |
BM-MSC |
Intravenous |
Well tolerated in moderate-to-severe CLAD, low-dose may slow progression of CLAD in some patients |
[11] |
4. Strategies to Improve MSC Delivery and Therapeutic Potential
4.1. MSC Delivery into the Donor Lung during EVLP
4.2. Genetically Engineered MSCs
References
- Chambers, D.C.; Perch, M.; Zuckermann, A.; Cherikh, W.S.; Harhay, M.O.; HayesJr, D.; Hsich, E.; Khush, K.K.; Potena, L.; Sadavarte, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transpl. 2021, 40, 1060–1072.
- Chacon-Alberty, L.; Fernandez, R.; Jindra, P.; King, M.; Rosas, I.; Hochman-Mendez, C.; Loor, G. Primary Graft Dysfunction in Lung Transplantation: A Review of Mechanisms and Future Applications. Transplantation 2023.
- Parulekar, A.D.; Kao, C.C. Detection, classification, and management of rejection after lung transplantation. J. Thorac. Dis. 2019, 11, S1732–S1739.
- Ohm, B.; Jungraithmayr, W. B Cell Immunity in Lung Transplant Rejection—Effector Mechanisms and Therapeutic Implications. Front. Immunol. 2022, 13, 845867.
- Venado, A.; Kukreja, J.; Greenland, J.R. Chronic Lung Allograft Dysfunction. Thorac. Surg. Clin. 2022, 32, 231–242.
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22.
- Galderisi, U.; Peluso, G.; Di Bernardo, G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2022, 18, 23–36.
- Mansourabadi, A.H.; Mohamed Khosroshahi, L.; Noorbakhsh, F.; Amirzargar, A. Cell therapy in transplantation: A comprehensive review of the current applications of cell therapy in transplant patients with the focus on Tregs, CAR Tregs, and Mesenchymal stem cells. Int. Immunopharmacol. 2021, 97, 107669.
- Vandermeulen, M.; Erpicum, P.; Weekers, L.; Briquet, A.; Lechanteur, C.; Detry, O.; Beguin, Y.; Jouret, F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation 2020, 104, 5.
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826.
- Erasmus, D.B.; Durand, N.; Alvarez, F.A.; Narula, T.; Hodge, D.O.; Zubair, A.C. Feasibility and Safety of Low-Dose Mesenchymal Stem Cell Infusion in Lung Transplant Recipients. Stem Cells Transl. Med. 2022, 11, 891–899.
- Keller, C.A.; Gonwa, T.A.; Hodge, D.O.; Hei, D.J.; Centanni, J.M.; Zubair, A.C. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl. Med. 2018, 7, 161–167.
- Chambers, D.C.; Enever, D.; Lawrence, S.; Sturm, M.J.; Herrmann, R.; Yerkovich, S.; Musk, M.; Hopkins, P.M. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 2017, 6, 1152–1157.
- Miller, C.L.; Jane, M.O.; Allan, J.S.; Madsen, J.C. Novel approaches for long-term lung transplant survival. Front. Immunol. 2022, 13, 931251.
- Doherty, D.F.; Roets, L.; Krasnodembskaya, A.D. The Role of Lung Resident Mesenchymal Stromal Cells in the Pathogenesis and Repair of Chronic Lung Disease. Stem Cells 2023, 41, 431–443.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Khan, R.S.; Newsome, P.N. A Comparison of Phenotypic and Functional Properties of Mesenchymal Stromal Cells and Multipotent Adult Progenitor Cells. Front. Immunol. 2019, 10, 1952.
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92.
- Wang, F.; Li, Y.; Wang, B.; Li, J.; Peng, Z. The safety and efficacy of mesenchymal stromal cells in ARDS: A meta-analysis of randomized controlled trials. Crit. Care 2023, 27, 31.
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140.
- Kawashima, M.; Juvet, S.C. The role of innate immunity in the long-term outcome of lung transplantation. Ann. Transl. Med. 2020, 8, 412.
- Manning, E.; Pham, S.; Li, S.; Vazquez-Padron, R.I.; Mathew, J.; Ruiz, P.; Salgar, S.K. Interleukin-10 delivery via mesenchymal stem cells: A novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum. Gene 2010, 21, 713–727.
- Wittwer, T.; Rahmanian, P.; Choi, Y.H.; Zeriouh, M.; Karavidic, S.; Neef, K.; Christmann, A.; Piatkowski, T.; Schnapper, A.; Ochs, M.; et al. Mesenchymal stem cell pretreatment of non-heart-beating-donors in experimental lung transplantation. J. Cardiothorac. Surg. 2014, 9, 151.
- La Francesca, S.; Ting, A.E.; Sakamoto, J.; Rhudy, J.; Bonenfant, N.R.; Borg, Z.D.; Cruz, F.F.; Goodwin, M.; Lehman, N.A.; Taggart, J.M.; et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: An initial pilot and feasibility study. Transpl. Res. 2014, 3, 19.
- McAuley, D.F.; Curley, G.F.; Hamid, U.I.; Laffey, J.G.; Abbott, J.; McKenna, D.H.; Fang, X.; Matthay, M.A.; Lee, J.W. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L809–L815.
- Tian, W.; Liu, Y.; Zhang, B.; Dai, X.; Li, G.; Li, X.; Zhang, Z.; Du, C.; Wang, H. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung 2015, 193, 85–95.
- Mordant, P.; Nakajima, D.; Kalaf, R.; Iskender, I.; Maahs, L.; Behrens, P.; Coutinho, R.; Iyer, R.K.; Davies, J.E.; Cypel, M.; et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J. Heart Lung Transpl. 2016, 35, 1245–1254.
- Watanabe, T.; Hoshikawa, Y.; Ishibashi, N.; Suzuki, H.; Notsuda, H.; Watanabe, Y.; Noda, M.; Kanehira, M.; Ohkouchi, S.; Kondo, T.; et al. Mesenchymal stem cells attenuate ischemia-reperfusion injury after prolonged cold ischemia in a mouse model of lung transplantation: A preliminary study. Surg. Today 2017, 47, 425–431.
- Schnapper, A.; Christmann, A.; Knudsen, L.; Rahmanian, P.; Choi, Y.H.; Zeriouh, M.; Karavidic, S.; Neef, K.; Sterner-Kock, A.; Guschlbauer, M.; et al. Stereological assessment of the blood-air barrier and the surfactant system after mesenchymal stem cell pretreatment in a porcine non-heart-beating donor model for lung transplantation. J. Anat. 2018, 232, 283–295.
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212.
- Martens, A.; Ordies, S.; Vanaudenaerde, B.M.; Verleden, S.E.; Vos, R.; Van Raemdonck, D.E.; Verleden, G.M.; Roobrouck, V.D.; Claes, S.; Schols, D.; et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res. 2017, 8, 159.
- Piatkowski, T.; Brandenberger, C.; Rahmanian, P.; Choi, Y.H.; Zeriouh, M.; Sabashnikov, A.; Wittwer, T.; Wahlers, T.C.W.; Ochs, M.; Mühlfeld, C. Localization of Exogenous Mesenchymal Stem Cells in a Pig Model of Lung Transplantation. Thorac. Cardiovasc. Surg. 2018, 66, 63–70.
- Wei, L.; Han, Z.J.; Xu, L.; Li, J.W. Effect of mesenchymal stem cells on expression of high mobility group box 1 protein in rats with ischemia reperfusion injury after lung transplantation. Zhonghua Yi Xue Za Zhi 2018, 98, 2019–2023.
- Nakajima, D.; Watanabe, Y.; Ohsumi, A.; Pipkin, M.; Chen, M.; Mordant, P.; Kanou, T.; Saito, T.; Lam, R.; Coutinho, R.; et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transpl. 2019, 38, 1214–1223.
- Pacienza, N.; Santa-Cruz, D.; Malvicini, R.; Robledo, O.; Lemus-Larralde, G.; Bertolotti, A.; Marcos, M.; Yannarelli, G. Mesenchymal Stem Cell Therapy Facilitates Donor Lung Preservation by Reducing Oxidative Damage during Ischemia. Stem Cells Int. 2019, 2019, 8089215.
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Maggioni, M.; Zanella, A.; Dondossola, D.; Fonsato, V.; Grange, C.; et al. Mesenchymal stem cell-derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transpl. 2019, 38, 1306–1316.
- Nykänen, A.I.; Mariscal, A.; Duong, A.; Estrada, C.; Ali, A.; Hough, O.; Sage, A.; Chao, B.T.; Chen, M.; Gokhale, H.; et al. Engineered mesenchymal stromal cell therapy during human lung ex vivo lung perfusion is compromised by acidic lung microenvironment. Mol. Methods Clin. Dev. 2021, 23, 184–197.
- Shimoyama, K.; Tsuchiya, T.; Watanabe, H.; Ergalad, A.; Iwatake, M.; Miyazaki, T.; Hashimoto, Y.; Hsu, Y.I.; Hatachi, G.; Matsumoto, K.; et al. Donor and Recipient Adipose-Derived Mesenchymal Stem Cell Therapy for Rat Lung Transplantation. Transpl. Proc. 2022, 54, 1998–2007.
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362.
- Lee, J.W.; Krasnodembskaya, A.; McKenna, D.H.; Song, Y.; Abbott, J.; Matthay, M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013, 187, 751–760.
- Ishibashi, N.; Watanabe, T.; Kanehira, M.; Watanabe, Y.; Hoshikawa, Y.; Notsuda, H.; Noda, M.; Sakurada, A.; Ohkouchi, S.; Kondo, T.; et al. Bone marrow mesenchymal stromal cells protect allograft lung transplants from acute rejection via the PD-L1/IL-17A axis. Surg. Today 2018, 48, 726–734.
- Khan, M.A.; Alanazi, F.; Ahmed, H.A.; Shamma, T.; Kelly, K.; Hammad, M.A.; Alawad, A.O.; Assiri, A.M.; Broering, D.C. iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res. 2019, 10, 290.
- Grove, D.A.; Xu, J.; Joodi, R.; Torres-Gonzales, E.; Neujahr, D.; Mora, A.L.; Rojas, M. Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation. J. Heart Lung Transpl. 2011, 30, 341–350.
- Guo, Z.; Zhou, X.; Li, J.; Meng, Q.; Cao, H.; Kang, L.; Ni, Y.; Fan, H.; Liu, Z. Mesenchymal stem cells reprogram host macrophages to attenuate obliterative bronchiolitis in murine orthotopic tracheal transplantation. Int. Immunopharmacol. 2013, 15, 726–734.
- Casey, A.; Dirks, F.; Liang, O.D.; Harrach, H.; Schuette-Nuetgen, K.; Leeman, K.; Kim, C.F.; Gerard, C.; Subramaniam, M. Bone marrow-derived multipotent stromal cells attenuate inflammation in obliterative airway disease in mouse tracheal allografts. Stem Cells Int. 2014, 2014, 468927.
- Watanabe, T.; Cypel, M.; Keshavjee, S. Ex vivo lung perfusion. J. Thorac. Dis. 2021, 13, 6602–6617.
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011, 364, 1431–1440.
- Tikkanen, J.M.; Cypel, M.; Machuca, T.N.; Azad, S.; Binnie, M.; Chow, C.W.; Chaparro, C.; Hutcheon, M.; Yasufuku, K.; de Perrot, M.; et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J. Heart Lung Transpl. 2015, 34, 547–556.
- Divithotawela, C.; Cypel, M.; Martinu, T.; Singer, L.G.; Binnie, M.; Chow, C.W.; Chaparro, C.; Waddell, T.K.; de Perrot, M.; Pierre, A.; et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg. 2019, 154, 1143–1150.
- Keller, M.; Bush, E.; Diamond, J.M.; Shah, P.; Matthew, J.; Brown, A.W.; Sun, J.; Timofte, I.; Kong, H.; Tunc, I.; et al. Use of donor-derived-cell-free DNA as a marker of early allograft injury in primary graft dysfunction (PGD) to predict the risk of chronic lung allograft dysfunction (CLAD). J. Heart Lung Transpl. 2021, 40, 488–493.
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transpl. 2015, 15, 2404–2412.
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737.
- Islam, D.; Huang, Y.; Fanelli, V.; Delsedime, L.; Wu, S.; Khang, J.; Han, B.; Grassi, A.; Li, M.; Xu, Y.; et al. Identification and Modulation of Microenvironment is Crucial for Effective MSC Therapy in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2019, 199, 1214–1224.
- Wang, C.; Lv, D.; Zhang, X.; Ni, Z.A.; Sun, X.; Zhu, C. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol. 2018, 37, 53–61.




