
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Sliwinska | -- | 3079 | 2023-06-29 12:51:36 | | | |
| 2 | Peter Tang | Meta information modification | 3079 | 2023-06-29 12:57:37 | | |
Video Upload Options
Despite significant progress in medicine, pancreatic cancer is one of the most tardily diagnosed cancer and is consequently associated with a poor prognosis and a low survival rate. The asymptomatic clinical picture and the lack of relevant diagnostic markers for the early stages of pancreatic cancer are believed to be the major constraints behind an accurate diagnosis of this disease. Furthermore, underlying mechanisms of pancreatic cancer development are still poorly recognized. It is well accepted that diabetes increases the risk of pancreatic cancer development, however the precise mechanisms are weakly investigated. Studies are focused on microRNAs as a causative factor of pancreatic cancer.
1. Introduction
2. microRNA—An Overview
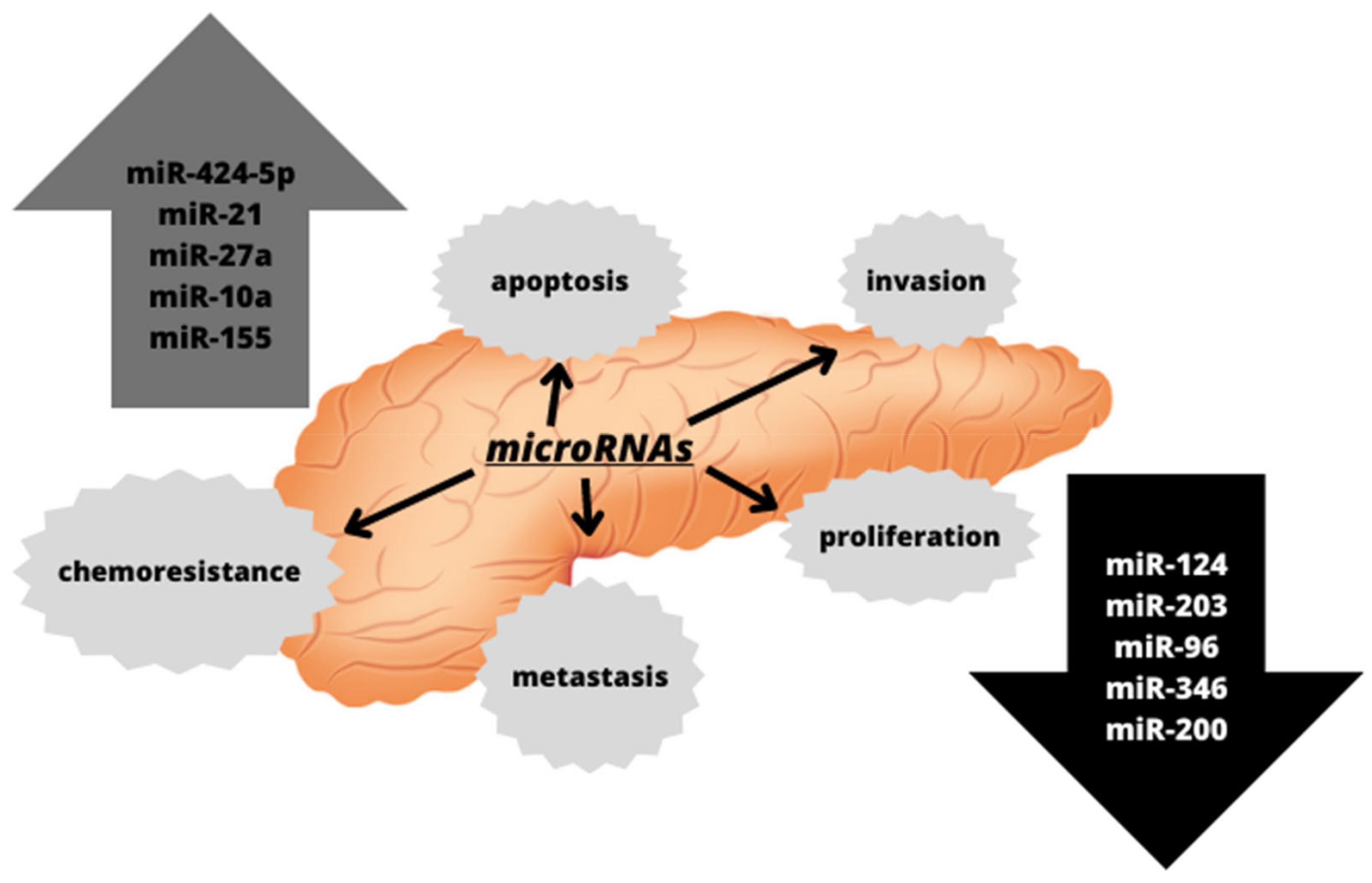
2.1. The Role of microRNAs in the Therapeutic Approaches
3. Diabetes and Pancreatic Cancer
|
Types of Diabetes |
Characteristics |
|---|---|
|
Type 1 insulin-dependent diabetes |
β-cell destruction and absolute insulin deficiency, onset most common in childhood, and it can be idiopathic as well as genetically determined. |
|
Type 2 non-insulin-dependent diabetes |
Insulin resistance, commonly associated with being overweight and obesity, and is most common in adults. |
|
Type 3 secondary diabetes |
It is a consequence of, among others, chronic or acute pancreatitis, pancreatectomy, pancreatic cancer, or other diseases, and is often confused with type II diabetes. |
|
Diabetes mellitus in pregnancy |
Usually occurs in the second and third trimesters of pregnancy, may persist beyond the end of pregnancy, and is associated with a significant risk to both the mother and newborn. |
|
Different types of diabetes: neonatal diabetes, MODY-type diabetes, diseases of the exocrine pancreas, drug-induced, and infection-related |
Drug-induced: some medicines, such as thiazides or pentamidines can destroy β-cells or lead to insulin resistance. MODY diabetes: occurs in young people, leads to impaired insulin secretion, and is inherited in an autosomal dominant manner. Neonatal diabetes: monogenic form, and can be both temporary and permanent. Infection-related: cytomegalovirus or Coxsackie B virus is able to induce type I diabetes. |
References
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Löhr, J.M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18.
- Poruk, K.E.; Firpo, M.A.; Adler, D.G.; Mulvihill, S.J. Screening for pancreatic cancer: Why, how, and who? Ann. Surg. 2013, 257, 17–26.
- Capasso, M.; Franceschi, M.; Rodriguez-Castro, K.I.; Crafa, P.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Leandro, G.; Meschi, T.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018, 89 (Suppl. S9), 141–146.
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11.
- Umans, D.S.; Hoogenboom, S.A.; Sissingh, N.J.; Lekkerkerker, S.J.; Verdonk, R.C.; van Hooft, J.E. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J. Gastroenterol. 2021, 27, 3148–3157.
- Kirkegård, J.; Gaber, C.; Lund, J.L.; Hinton, S.P.; Ladekarl, M.; Heide-Jørgensen, U.; Cronin-Fenton, D.; Mortensen, F.V. Acute pancreatitis as an early marker of pancreatic cancer and cancer stage, treatment, and prognosis. Cancer Epidemiol. 2020, 64, 101647.
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147.
- Tüfekci, K.U.; Meuwissen, R.L.; Genç, S. The role of microRNAs in biological processes. Methods Mol. Biol. 2014, 1107, 15–31.
- Zhao, Y.; Fan, S.; Yuan, P.; Li, G. Expression characteristics and interaction networks of microRNAs in spleen tissues of grass carp (Ctenopharyngodon idella). PLoS ONE 2022, 17, e0266189.
- Tan, G.S.; Garchow, B.G.; Liu, X.; Metzler, D.; Kiriakidou, M. Clarifying mammalian RISC assembly in vitro. BMC Mol. Biol. 2011, 12, 19.
- Lin, S.; Gregory, R. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Hashimoto, Y.; Akiyama, Y.; Yuasa, Y. Multiple-to-multiple relationships between microRNAs and target genes in gastric cancer. PLoS ONE 2013, 8, e62589.
- Barman, S.; Fatima, I.; Singh, A.B.; Dhawan, P. Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 4765.
- Sempere, L.F.; Powell, K.; Rana, J.; Brock, A.A.; Schmittgen, T.D. Role of non-coding RNAs in tumor progression and metastasis in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 761–776.
- Morris, J.P., IV; Greer, R.; Russ, H.A.; von Figura, G.; Kim, G.E.; Busch, A.; Lee, J.; Hertel, K.J.; Kim, S.; McManus, M.; et al. Dicer Regulates Differentiation and Viability during Mouse Pancreatic Cancer Initiation. PLoS ONE 2014, 9, e95486.
- Lynn, F.C.; Skewes-Cox, P.; Kosaka, Y.; McManus, M.T.; Harfe, B.D.; German, M.S. MicroRNA Expression Is Required for Pancreatic Islet Cell Genesis in the Mouse. Diabetes 2007, 56, 2938–2945.
- Su, Y.-H.; Hsu, T.-W.; Chen, H.-A.; Su, C.-M.; Huang, M.-T.; Chuang, T.-H.; Su, J.L.; Hsieh, C.-L.; Chiu, C.-F. ERK-mediated transcriptional activation of Dicer is involved in gemcitabine resistance of pancreatic cancer. J. Cell Physiol. 2021, 236, 4420–4434.
- Solarski, M.; Rotondo, F.; Foulkes, W.D.; Priest, J.R.; Syro, L.V.; Butz, H.; Cusimano, M.D.; Kovacs, K. DICER1 gene mutations in endocrine tumors. Endocr. Relat. Cancer 2018, 25, R197–R208.
- Park, J.M.; Peng, J.M.; Shen, Y.S.; Lin, C.Y.; Hsu, T.W.; Su, Y.H.; Chen, H.A.; Saengboonmee, C.; Chang, J.S.; Chiu, C.F.; et al. Phosphomimetic Dicer S1016E triggers a switch to glutamine metabolism in gemcitabine-resistant pancreatic cancer. Mol. Metab. 2022, 65, 101576.
- Elgheznawy, A.; Shi, L.; Hu, J.; Wittig, I.; Laban, H.; Pircher, J.; Mann, A.; Provost, P.; Randriamboavonjy, R.; Fleming, I. Dicer Cleavage by Calpain Determines Platelet microRNA Levels and Function in Diabetes. Circ. Res. 2015, 117, 157–165.
- Gironella, M.; Seux, M.; Xie, M.J.; Cano, C.; Tomasini, R.; Gommeaux, J.; Garcia, S.; Nowak, J.; Yeung, M.L.; Jeang, K.T.; et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc. Natl. Acad. Sci. USA 2007, 104, 16170–16175.
- Farasati Far, B.; Vakili, K.; Fathi, M.; Yaghoobpoor, S.; Bhia, M.; Naimi-Jamal, M.R. The role of microRNA-21 (miR-21) in pathogenesis, diagnosis, and prognosis of gastrointestinal cancers: A review. Life Sci. 2022, 316, 121340.
- Rawat, M.; Kadian, K.; Gupta, Y.; Kumar, A.; Chain, P.S.G.; Kovbasnjuk, O.; Kumar, S.; Parasher, G. MicroRNA in Pancreatic Cancer: From Biology to Therapeutic Potential. Genes 2019, 10, 752.
- Srivastava, S.K.; Arora, S.; Singh, S.; Bhardwaj, A.; Averett, C.; Singh, A.P. MicroRNAs in pancreatic malignancy: Progress and promises. Cancer Lett. 2014, 347, 167–174.
- Halkova, T.; Cuperkova, R.; Minarik, M.; Benesova, L. MicroRNAs in Pancreatic Cancer: Involvement in Carcinogenesis and Potential Use for Diagnosis and Prognosis. Gastroenterol. Res. Pract. 2015, 2015, 892903.
- Wu, J.S.; Jiang, J.; Chen, B.J.; Wang, K.; Tang, Y.L.; Liang, X.H. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899.
- Weiss, F.U.; Marques, I.J.; Woltering, J.M.; Vlecken, D.H.; Aghdassi, A.; Partecke, L.I.; Heidecke, C.D.; Lerch, M.M.; Bagowski, C.P. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 2009, 137, 2136–2145.e1–7.
- Takahashi, R.U.; Prieto-Vila, M.; Kohama, I.; Ochiya, T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019, 110, 1140–1147.
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502.
- Stenvang, J.; Petri, A.; Lindow, M.; Obad, S.; Kauppinen, S. Inhibition of microRNA function by antimiR oligonucleotides. Silence 2012, 3, 1.
- Guo, S.; Fesler, A.; Huang, W.; Wang, Y.; Yang, J.; Wang, X.; Zheng, Y.; Hwang, G.R.; Wang, H.; Ju, J. Functional Significance and Therapeutic Potential of miR-15a Mimic in Pancreatic Ductal Adenocarcinoma. Mol. Ther. Nucleic. Acids 2020, 19, 228–239.
- Imamura, T.; Komatsu, S.; Ichikawa, D.; Miyamae, M.; Okajima, W.; Ohashi, T.; Nishibeppu, K.; Konishi, H.; Shiozaki, A.; Morimura, R.; et al. Depleted tumor suppressor miR-107 in plasma relates to tumor progression and is a novel therapeutic target in pancreatic cancer. Sci. Rep. 2017, 7, 5708.
- Meijer, L.L.; Garajová, I.; Caparello, C.; Le Large, T.Y.S.; Frampton, A.E.; Vasile, E.; Funel, N.; Kazemier, G.; Giovannetti, E. Plasma miR-181a-5p Downregulation Predicts Response and Improved Survival After FOLFIRINOX in Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2020, 271, 1137–1147.
- International Diabetes Federation—IDF. Diabetes Atlas 2022 Reports. Available online: https://diabetesatlas.org/2022-reports/ (accessed on 21 April 2016).
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2019) . Wien. Klin. Wochenschr. 2019, 131 (Suppl. S1), 6–15.
- Gopalan, A.; Mishra, P.; Alexeeff, S.E.; Blatchins, M.A.; Kim, E.; Man, A.H.; Grant, R.W. Prevalence and predictors of delayed clinical diagnosis of Type 2 diabetes: A longitudinal cohort study. Diabet. Med. 2018, 35, 1655–1662.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33.
- Wang, M.; Hng, T.M. HbA1c: More than just a number. Aust. J. Gen. Pract. 2021, 50, 628–632.
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546.




