One of the most common gastrointestinal cancers apart from stomach or colorectal cancer is pancreatic cancer. Considering the localization in the pancreas, it is divided into two types, the most commonly occurring exocrine pancreatic cancer, comprising adenocarcinoma, and neuroendocrine pancreatic cancer, respectively [
1]. Despite significant progress in medicine, pancreatic cancer is detected at its advanced stages resulting in a poor prognosis for patients. The largest percentage of diagnosed patients refers to advanced stages with distant metastases, and for these patients a five-year survival rate is less than 5% and a median time from diagnosis is equivalent to around six months [
2,
3]. Pancreatic cancer risk factors include age, family history, and gene mutations, such as in the
BRCA2 gene, or hereditary syndromes including hereditary pancreatitis [
4]. Another risk factor is lifestyle, which is a common factor in the development of both diabetes and pancreatic cancer, with diabetes itself also carrying the risk of developing cancer. An unhealthy lifestyle includes alcohol drinking, smoking, and diet, thereby leading to the development of obesity. Both acute and chronic pancreatitis are crucial risk factors of pancreatic cancer development. Pancreatitis as an inflammatory disease is a source of chronic inflammation leading to pancreatic calcifications and exocrine insufficiency [
5]. Acute pancreatitis may constitute an early symptom of pancreatic cancer [
6]. Chronic pancreatitis is accompanied with the downregulation of the tumor-suppressing genes, such as
p16,
TP53, and
SMAD4 (mothers against decapentaplegic homolog), respectively, along with the upregulation of the oncogenic
KRAS (Kirsten rat sarcoma virus),
TNF-α (tumor necrosis factor alpha), and
NF-kB (nuclear factor kappa B) genes [
5].
2. microRNA—An Overview
MicroRNAs are non-coding, single-stranded small RNAs, with an average length of 19–25 nucleotides. They are involved in many biological processes, such as cell growth, proliferation, differentiation, and organogenesis, and their main mechanism of action is to regulate gene function either by the degradation of mRNA or through the inhibition of mRNA translation [
51].
The biogenesis of microRNA consists of a series of processes occurring in both the nucleus and in the cytoplasm, and genes for microRNAs are found in both exons, introns, and untranslated sequences, thereby allowing for the simultaneous formation of microRNA transcripts and mRNAs [
52]. One of the first stages of biogenesis is the post-transcriptional formation of primary transcripts (pri-miRNA) with a cap at the 5’ end and a poly(A) tail, which are then capped and spliced by the nuclear protein DGCR8 (DiGeorge syndrome critical region) combined with the Drosha enzyme which contains two RNase III domains, each of which cleaves one strand of the dsRNA [
53]. The pri-miRNA formed has a characteristic hairpin-shaped structure and transport proteins such as Exportin 5 and is then transported from the nucleus to the cytoplasm, where the next step in microRNA formation takes place. The processing of the pre-miRNA is performed by the enzyme Dicer and involves the cutting and removing of the terminal loop, resulting in the formation of a mature microRNA–microRNA duplex, in which one strand is the guide strand and the other is the passenger strand, respectively [
52,
53]. The Argonaute family proteins are the core components of the RNA-induced silencing complex (RISC), which attaches to the mature microRNA, and this activated complex is able to cause the repression of the translation and the degradation of the target mRNA [
52,
54]. Both the leading and passenger strands are able to participate in the regulation of expression such that one of the microRNA strands is complementary to the target mRNA, and this involves the negative regulation of gene expression at the post-transcriptional level [
53]. The complementarity between the microRNA and mRNA does not have to be complete, meaning one microRNA could regulate many genes as its targets, and reversely one gene may be targeted by many microRNAs [
55].
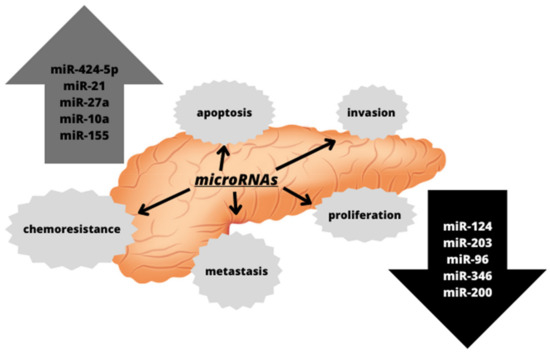
microRNAs are involved in the regulation of many physiological processes required for the function of the organism, including differentiation and apoptosis. However, they are also involved in the processes associated with the development of several diseases, such as angiogenesis or carcinogenesis. In the case of carcinogenesis, the changes in the expression of microRNAs during this process have been observed, and the diversity of the genes regulated by microRNAs makes it possible for them to behave both as oncogenes and as transformation suppressors [
50]. The alterations in the expression of various microRNAs have also been observed in pancreatic cancers, as depicted in
Figure 1. They have been associated with pancreatic cancer progression and metastasis, suggesting that microRNA expression profiling may serve as both diagnostic and prognostic biomarkers [
56].
Figure 1. Altered microRNAs detected in pancreatic cancer and their impact on cellular processes associated with carcinogenesis.
Studies have shown that Dicer, involved in the biogenesis and activity of microRNA, participates in pancreatic cancer development. Dicer’s function is important throughout the development of the pancreas, as it affects the endocrine functions of this organ [
57,
58]. Studies conducted on mice in which knockout Dicer was used revealed that these animals had a radical reduction in the ventral pancreas, and a decrease in the overall epithelial contribution to the dorsal pancreas [
58]. Staining for specific hormones showed a significant loss of β-cells responsible for insulin production [
58]. In cancers, including pancreatic cancer, there is an altered expression of various microRNAs, and this may be due to the altered expression of the enzyme Dicer by targeting ERK/Sp1 signaling, which can directly contribute to the initiation, growth, and progression of the tumor [
59]. Research conducted on mice have shown that the reduced or even complete knockout of expression of Dicer accelerates KRAS-driven acinar dedifferentiation and leads to the loss in the polarity of cluster cells, which initiates both epithelial-to-mesenchymal transitions (EMT) and cluster metaplasia to ductal, a process that has been shown to precede and promote the formation of pancreatic cancer precursors [
60]. Other studies have shown a positive correlation between the high expression of the enzyme Dicer and resistance to gemcitabine, a cytostatic drug used in cancer therapy [
59,
61]. Interestingly, study conducted on platelets collected from patients with diabetes revealed that Dicer levels were significantly reduced compared to the healthy patients (79 ± 3%), what may suggest an association between diabetes and pancreatic cancer, but there is still too little data on this issue [
62].
One of the activities that microRNAs exhibit in tumorigenesis is their participation in initiation stage through dysregulation of apoptosis, also termed as programmed cell death. Under physiological conditions, in response to DNA damage, either DNA repair or apoptosis is induced, and thus cellular homeostasis is maintained. It was demonstrated that microRNAs participate in the dysregulation of apoptosis in cancer. One of them is miR-155, that overexpression in pancreatic cancer resulted in the down-regulation of p53 protein, thereby inhibiting apoptosis [
36].
The next stage of carcinogenesis is tumor progression stage connected with dysregulation of the cell cycle. The later one is regulated by numerous checkpoints, oncogenes, and tumor suppressors. In cancer, excessive cell proliferation is observed, which is associated, among others, with the overexpression of oncogenic microRNAs, such as miR-21. Its upregulation negatively affects the expression of tumor suppressors, such as
PTEN (Phosphatase and Tensin Homolog),
SMAD7 (mothers against decapentaplegic homolog),
PDCD4 (programmed cell death 4), or
KRAS, which are involved in the essential pathways connected with the regulation of the proliferation, growth, and transformation of epithelial cells [
63]. Another microRNA related with increased cell proliferation is miR-424-5p, whose up-expression was observed in pancreatic cancer, evoked the decreases in SOC6 (suppressor of cytokine signaling) protein levels and increased ERK (extracellular signal-regulated kinase) pathway activity [
64]. miR-27a, an oncogenic microRNA, is also significantly upregulated in pancreatic cancer, and its inhibition has been shown to reduce tumor cell growth and proliferation by inducing the tumor suppressor Spry2 (Sprouty) [
65]. It has been observed that in pancreatic cancers there is also the downregulation of several suppressor microRNAs, including miR-124, miR-203, and miR-96. miR-124 regulates the expression of the
RAC1 (Rac family small GTPase 1) oncogene and inhibits tumor cell proliferation. However, it has been shown that in pancreatic cancer reduced expression of miR-124 interferes with this process, and it was associated with a poor survival among patients with pancreatic cancer [
63]. In turn, the decreased expression of miR-203 leads to the transition to the G1 phase of the cell cycle, and consequently increases the proliferation of the cancer cells [
66].
Another important role that can be attributed to microRNAs is their involvement in the regulation of invasion and the formation of metastases. One of the elements of cancer cell biology is their invasiveness, which is the ability to migrate from the original tumor mass and then enter the bloodstream or lymphatic system to settle in a new, distant place and proliferate from there in an uncontrolled way, thereby creating metastases [
67]. Such metastases significantly reduce the prognosis for patients and contribute to an increased mortality. The key step in invasion and metastasis is the epithelial-to-mesenchymal transition, which enables cancer cells to migrate. microRNAs are also engaged in the EMT transition. Upregulation of miR-10a was found to promote pancreatic cancer metastasis, and this was found to be caused by the decrease in the expression of the HOXB1 (homeobox protein) and HOXB3 genes, which encode a highly conserved family of transcription factors necessary for the proper course of morphogenesis [
68]. Another microRNA associated with metastasis is miR-34b, which negatively regulates the expression of Smad3 protein, the metastasis promoter. Reduced levels of this microRNA was observed in pancreatic cancers [
64]. It has also been shown that members of the miR-200 family and the ZEB1 (zinc finger E-box binding homeobox) and SIP1 (Smad-interacting protein-1) proteins regulate each other to form a feedback loop, thereby controlling the epithelial-to-mesenchymal transition. The decrease in the expression of the miR-200 family was found to induce the EMT [
66].
The Role of microRNAs in the Therapeutic Approaches
The utility of microRNAs in therapeutic approaches are based on several of their features. Firstly, microRNAs are smaller than proteins, meaning they can be easily introduced into the cell and also easily isolate from the biological samples, such as serum, saliva, or milk [
69]. Secondly, microRNAs are secreted by different cell types and are transported to the target site by extracellular vesicles, meaning they are able to regulate the tumor microenvironment as a result. Thus, knowing the biology and the mechanisms of action of microRNAs, the therapeutic approach focuses primarily on either inhibiting the oncogenic microRNAs, or restoring the expression of suppressor microRNAs. Another therapeutic approach is the use of anti-miR oligonucleotides, which sequester the mature microRNA leading to the direct functional inhibition of the microRNA and the depression of their targets [
70,
71]. These features have made these non-coding RNAs as an interesting target for many scientists and clinicians.
The use of microRNAs in therapy was demonstrated by Guo et al. [
72]. miR-15 acts as a tumor suppressor and alters cell cycle control.
In vivo studies have shown that miR-15 in combination with 5-FU improves survival, either alone or in combination with gemcitabine [
72]. In in vitro studies conducted on three pancreatic cancer cell lines (AsPC-1, PANC-1, and Hs 766T, respectively), the 5-FU-miR-15 complex was found to inhibit the proliferation of these pancreatic cancer cells, and additionally sensitize the cells to gemcitabine, which thereby enhanced the therapeutic effects [
72]. The effects of miR-15a are mediated through the regulation of several important target genes, including checkpoint kinase 1 (
Chk1) and
Yap-1 [
72]. Both genes are elevated in pancreatic ductal adenocarcinoma and are good target candidates for therapeutic development in PDAC (pancreatic ductal adenocarcinoma) [
72]. The administration of miR-15 with 5-FU resulted in the inclusion of the 5-FU-miR-15 complex in the RISC complex, and the suppression of Yap-1 and Chk1 resulting in cell cycle arrest, apoptosis, and the attenuation of EMT [
72]. To assess how the 5-FU-miR-15 complex affected metastasis and chemoresistance, researchers have created mouse models of pancreatic cancer metastasis and then implemented therapy with 5-FU-miR-15. The results showed that this complex significantly inhibited the growth of a metastatic tumor at a dose fifteen times lower than gemcitabine, and the inhibitory effect was further enhanced in combination with gemcitabine [
72]. In addition, Guo et al. found that resistance developed in a group of mice treated with gemcitabine alone, but not in mice treated with the 5-FU-miR-15 complex, which was connected with a significantly longer survival [
72].
microRNAs can also be used to monitor the course of therapy. An example of such a microRNA is miR-107, which under physiological conditions is a tumor suppressor. However, its reduced levels were identified in the plasma of people with pancreatic cancer. A study by Imamura et al. found that following surgery to remove the pancreatic tumor, miR-107 level increased significantly, which was associated with health improvement [
73]. Meijer et al. demonstrated the effectiveness of miR-181a-5p in monitoring FOLFIRINOX therapy in patients with pancreatic cancer. After the treatment, there was a significant decrease in miR-181a-5p in the plasma, which was associated with a better prognosis for patients [
74].
Currently, the use of microRNAs in treatments for pancreatic cancer are not approved for use, and clinical trials are underway to see whether microRNAs can be utilized in the detection and therapy of both pancreatic cancer. These attempts include the creation of a biobank of pancreatic cancer tissue and plasma from patients suffering from pancreatic cancer, pancreatitis, and normal pancreas for early detection. However, despite positive data from the experimental treatment of pancreatic cancer with microRNAs, there are still too few studies to include microRNA in the therapy as an agent or as a marker for therapy monitoring.
3. Diabetes and Pancreatic Cancer
Diabetes is one of the civilizational diseases characterized by a constantly growing number of incidences. According to the World Diabetes Federation (IDF) data from 2021, 537 million adults live with diabetes (often undiagnosed), which is 1 person per 10 individuals, and by 2030 this number will be expected to increase to 643 million [
75]. Diabetes and its late diagnosis are not only burdensome for the patient but is also a burden financially for health care systems, as it is associated with a significant increase in the costs of diabetes and its complicative treatment. According to the generally accepted definition, diabetes mellitus is defined as a group of heterogeneous metabolic disorders which are caused by an impaired or absent insulin secretion, with a common feature being elevated blood glucose levels [
76]. In most people with diabetes, clinical diagnosis occurs following a long delay, which is associated with the fact that the initial period of the disease is asymptomatic, and progressive hyperglycemia causes not only microvascular changes, but also contributes to both morphological and functional changes in tissues, leading to the impairment of the key organs [
77]. Diabetes mellitus is divided into several types based on the mechanism of development, age of onset, and method of treatment, as shown in
Table 1.
Table 1. Types of diabetes and their characteristics, based on the World Health Organization Classification of Diabetes Mellitus, 2019.
|
Types of Diabetes
|
Characteristics
|
|
Type 1 insulin-dependent diabetes
|
β-cell destruction and absolute insulin deficiency, onset most common in childhood, and it can be idiopathic as well as genetically determined.
|
|
Type 2 non-insulin-dependent diabetes
|
Insulin resistance, commonly associated with being overweight and obesity, and is most common in adults.
|
|
Type 3 secondary diabetes
|
It is a consequence of, among others, chronic or acute pancreatitis, pancreatectomy, pancreatic cancer, or other diseases, and is often confused with type II diabetes.
|
|
Diabetes mellitus in pregnancy
|
Usually occurs in the second and third trimesters of pregnancy, may persist beyond the end of pregnancy, and is associated with a significant risk to both the mother and newborn.
|
|
Different types of diabetes: neonatal diabetes, MODY-type diabetes, diseases of the exocrine pancreas, drug-induced, and infection-related
|
Drug-induced: some medicines, such as thiazides or pentamidines can destroy β-cells or lead to insulin resistance.
MODY diabetes: occurs in young people, leads to impaired insulin secretion, and is inherited in an autosomal dominant manner.
Neonatal diabetes: monogenic form, and can be both temporary and permanent.
Infection-related: cytomegalovirus or Coxsackie B virus is able to induce type I diabetes.
|
Diabetes mellitus is diagnosed when fasting plasma glucose (FPG) is ≥126 mg/dl or following the oral glucose tolerance test (OGTT) showing blood glucose levels of ≥200 mg/dl, respectively [
78]. Another diagnostic tool is the measurement of HbA1c (≥48 mmol/mol), i.e., glycated hemoglobin, which is also used to control the metabolic compliance in diabetic patients. It reflects an average glycemia over a period of 3 months [
79].
In diabetes, there is no universal schedule of treatment due to the different types and forms of this disease. In type 1 diabetes, the most common form of treatment to control glycemia is the use of insulin in the form of injections or special insulin pumps. In type 2 diabetes, treatment begins with changes in nutritional habits and physical activity, as this type of diabetes is associated with lifestyle-related obesity. Type 2 diabetes patients frequently have insulin resistance, and if these lifestyle changes do not improve glycemia, patients are then treated with metformin, which is the most commonly used oral hypoglycemic drug. Metformin is the first-line drug, which inhibits glucose production in the liver and increases insulin action in the muscles [
80]. Other drugs are also used, such as α-glucosidase inhibitors, which prolong the time of the digestion of carbohydrates, incretins that reduce glucagon secretion, or SGLT-2 (sodium-glucose cotransporter), which contributes to the elimination of glucose with the urine and thereby lower blood glucose levels [
80]. In other types of diabetes, therapy is matched to the patients, and depends, among other things, on comorbidities or the patient’s state of health.