
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jyoti C Patel | -- | 3306 | 2023-06-27 23:28:02 | | | |
| 2 | Rita Xu | -13 word(s) | 3293 | 2023-06-28 05:06:20 | | | | |
| 3 | Rita Xu | + 60 word(s) | 3353 | 2023-06-29 03:26:04 | | | | |
| 4 | Rita Xu | Meta information modification | 3353 | 2023-06-30 07:33:52 | | | | |
| 5 | Jyoti C Patel | -58 word(s) | 3295 | 2023-06-30 20:59:27 | | |
Video Upload Options
Insulin crosses the blood–brain barrier to enter the brain from the periphery. In the brain, insulin has well-established actions as a satiation signal in the hypothalamus, as well as effects on feeding at the level of mesolimbic dopamine neurons in the midbrain. However, insulin also acts in the striatum, a forebrain region that is crucial for movement, mood and motivated behavior. The striatum shows abundant expression of insulin receptors (InsRs) throughout. These receptors are found on interneurons and striatal projections neurons, as well as on glial cells and dopamine axons.
1. Introduction
2. Insulin Promotes Striatal Dopamine Release through ChIs and nAChRs
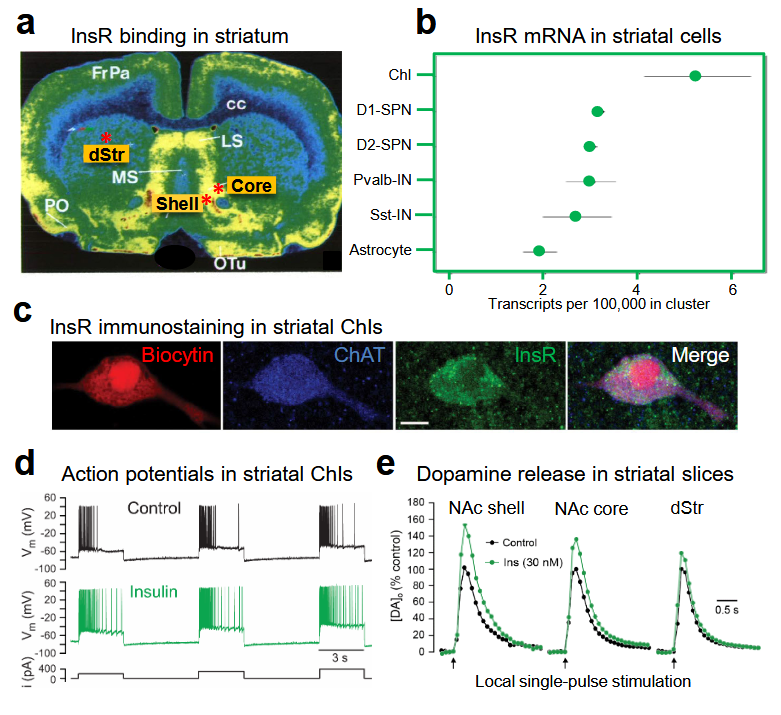
2.1. Insulin Increases the Excitability of Striatal ChIs
2.2. Insulin Boosts Dopamine Release via InsRs, and Requires PI3K and nAChRs
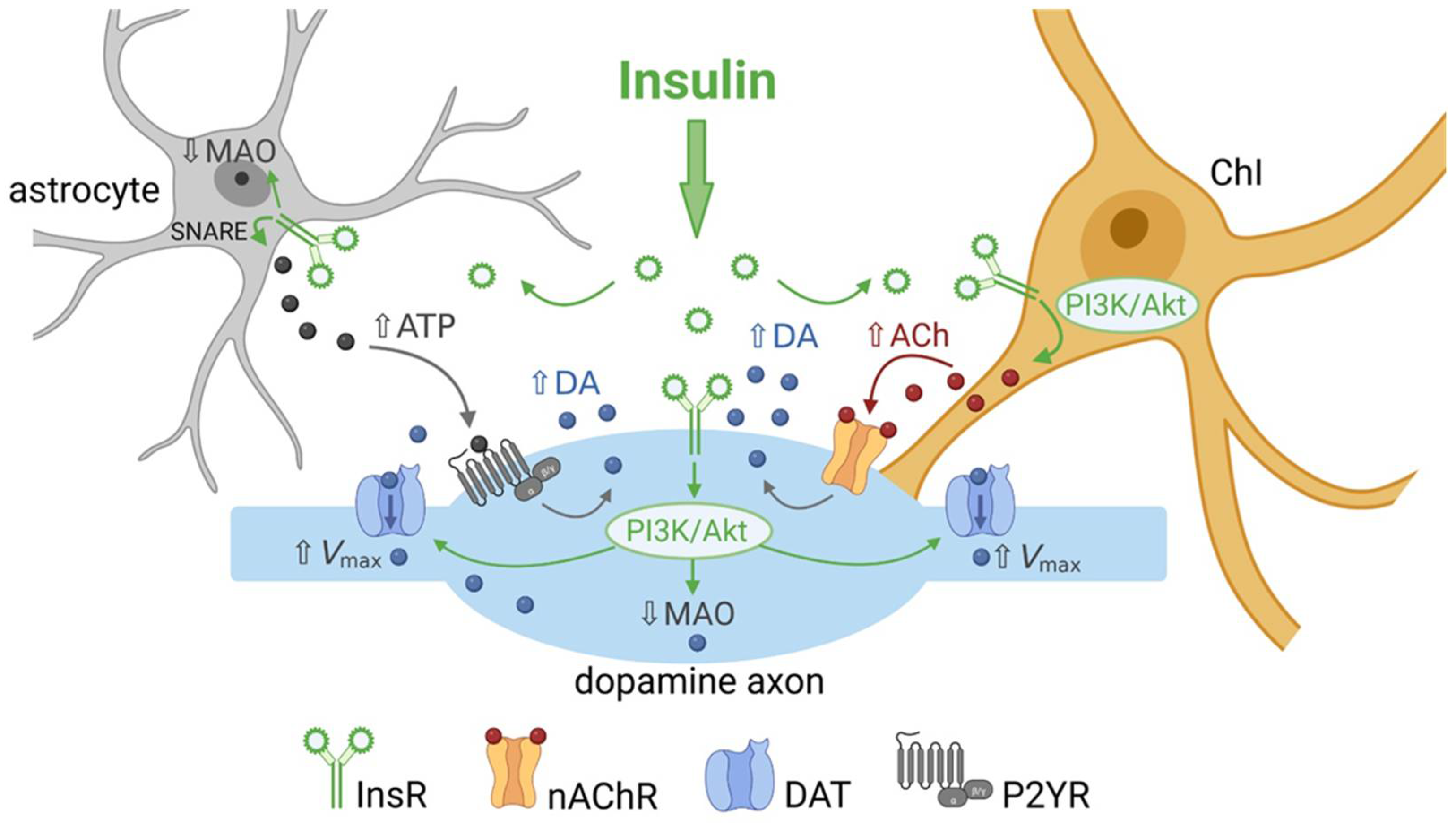
3. Insulin Enhances Striatal Dopamine Uptake via DAT on Dopamine Axons
4. Insulin Inhibits Dopamine Metabolism
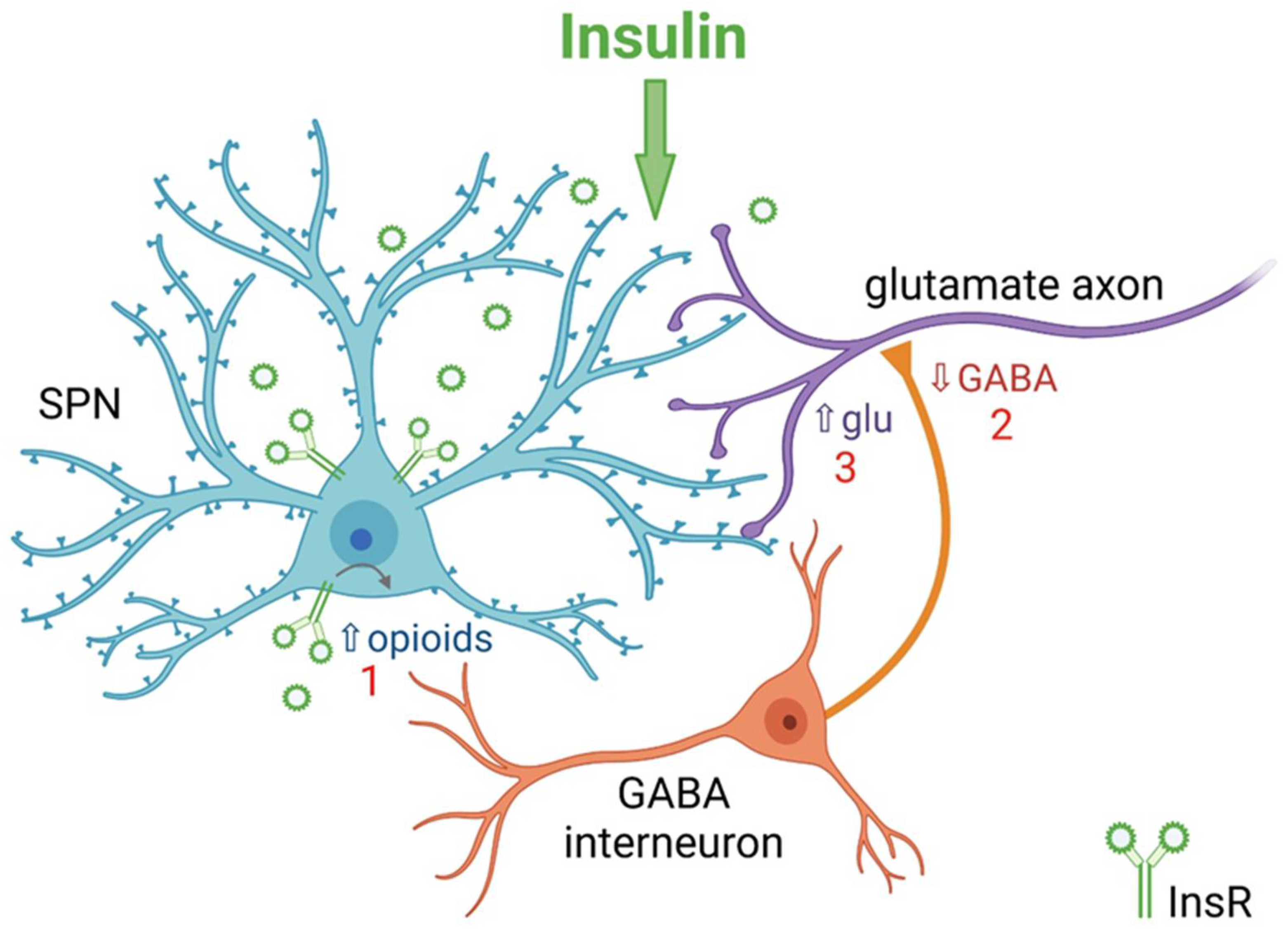
5. Insulin Bidirectionally Alters the Excitatory Regulation of Spiny Projection Neurons (SPNs)
6. Insulin Actions on Striatal Glial Cells
7. Conclusions
It is clear that insulin’s actions in the striatum are multifaceted in terms of the striatal elements involved and which insulin-sensitive receptors mediate the effects. Insulin can boost dopamine release indirectly through actions at InsRs on ChIs and astrocytes, with slower regulation by inhibiting dopamine metabolism by MAO. In addition, insulin actions on SPNs that engage local striatal microcircuits can have a direct influence on striatal and basal ganglia output. Information about the behavioral impact of striatal insulin signaling is limited. However, the few studies available have provided valuable evidence indicating an important role for striatal insulin beyond its role as a satiation/satiety signal.
References
- Dagen, M.M. History of Insulin. In Encyclopedia of Life Sciences (eLS); John Wiley & Sons, Ltd.: Chichester, UK, 2016.
- Banks, W.A. The source of cerebral insulin. Eur. J. Pharmacol. 2004, 490, 5–12.
- Banks, W.A.; Owen, J.B.; Erickson, M.A. Insulin in the brain: There and back again. Pharmacol. Ther. 2012, 136, 82–93.
- Gray, S.M.; Barrett, E.J. Insulin transport into the brain. Am. J. Physiol. Cell. Physiol. 2018, 315, C125–C136.
- Nakabeppu, Y. Origins of brain insulin and its function. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; pp. 1–11.
- Mazucanti, C.H.; Liu, Q.R.; Lang, D.; Huang, N.; O’Connell, J.F.; Camandola, S.; Egan, J.M. Release of insulin produced by the choroid plexis is regulated by serotonergic signaling. JCI Insight 2019, 4, e131682.
- Molnár, G.; Faragó, N.; Kocsis, Á.K.; Rózsa, M.; Lovas, S.; Boldog, E.; Báldi, R.; Csajbók, É.; Gardi, J.; Puskás, L.G.; et al. GABAergic neurogliaform cells represent local sources of insulin in the cerebral cortex. J. Neurosci. 2014, 34, 1133–1137.
- Csajbók, É.A.; Kocsis, Á.K.; Faragó, N.; Furdan, S.; Kovács, B.; Lovas, S.; Molnár, G.; Likó, I.; Zvara, Á.; Puskás, L.G.; et al. Expression of GLP-1 receptors in insulin-containing interneurons of rat cerebral cortex. Diabetologia 2019, 62, 717–725.
- Pitt, J.; Wilcox, K.C.; Tortelli, V.; Diniz, L.P.; Oliveira, M.S.; Dobbins, C.; Yu, X.W.; Nandamuri, S.; Gomes, F.C.A.; DiNunno, N.; et al. Neuroprotective astrocyte-derived insulin/insulin-like growth factor 1 stimulates endocytic processing and extracellular release of neuron-bound Aβ oligomers. Mol. Biol. Cell 2017, 28, 2623–2636.
- Strubbe, J.H.; Porte, D., Jr.; Woods, S.C. Insulin responses and glucose levels in plasma and cerebrospinal fluid during fasting and refeeding in the rat. Physiol. Behav. 1988, 44, 205–208.
- Banks, W.A.; Kastin, A.J. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides 1998, 19, 883–889.
- Havrankova, J.; Schmechel, D.; Roth, J.; Brownstein, M. Identification of insulin in rat brain. Proc. Natl. Acad. Sci. USA 1978, 75, 5737–5741.
- Woods, S.C.; Seeley, R.J.; Baskin, D.G.; Schwartz, M.W. Insulin and the blood brain barrier. Curr. Pharm. Des. 2003, 9, 795–800.
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829.
- Hill, J.M.; Lesniak, M.A.; Pert, C.B.; Roth, J. Autoradiographic localization of insulin receptors in rat brain: Prominence in olfactory and limbic areas. Neuroscience 1986, 17, 1127–1138.
- Werther, G.A.; Hogg, A.; Oldfield, B.J.; McKinley, M.J.; Figdor, R.; Allen, A.M.; Mendelsohn, F.A. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 1987, 121, 1562–1570.
- Schulingkamp, R.J.; Pagano, T.C.; Hung, D.; Raffa, R.B. Insulin receptors and insulin action in the brain: Review and clinical implications, Neurosci. Biobehav. Rev. 2000, 24, 855–872.
- Figlewicz, D.P.; Evans, S.B.; Murphy, J.; Hoen, M.; Baskin, D.G. Expression of receptors for insulin and leptin in ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003, 964, 107–115.
- Stouffer, M.A.; Woods, C.A.; Patel, J.C.; Lee, C.R.; Witkovsky, P.; Bao, L.; Machold, R.P.; Jones, K.T.; de Vaca, S.C.; Reith, M.E.; et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat. Commun. 2015, 6, 8543.
- Figlewicz, D.P. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat: Historical perspective. Brain Res. 2016, 1645, 68–70.
- Bondy, C.A.; Cheng, C.M. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 2004, 490, 25–31.
- Vigneri, R.; Squatrito, S.; Sciacca, L. Insulin and its analogs: Actions via insulin and IGF receptors. Acta. Diabetol. 2010, 47, 271–278.
- Bevan, P. Insulin signalling. J. Cell Sci. 2001, 114, 1429–1430.
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96.
- Boucher, J.; Tseng, Y.H.; Kahn, C.R. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J. Biol. Chem. 2010, 285, 17235–17245.
- Siddle, K. Signalling by insulin and IGF receptors: Supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10.
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D. Central nervous system control of food intake. Nature 2000, 404, 661–671.
- Bellisle, F.; Drewnowski, A.; Anderson, G.H.; Westerterp-Plantenga, M.; Martin, C.K. Sweetness, satiation, and satiety. J. Nutr. 2012, 142, 1149S–1154S.
- Tiedemann, L.J.; Schmid, S.M.; Hettel, J.; Giesen, K.; Francke, P.; Büchel, C.; Brassen, S. Central insulin modulates food valuation via mesolimbic pathways. Nat. Commun. 2017, 8, 16052.
- Ferrario, C.R.; Reagan, L.P. Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. Neuropharmacology 2018, 136, 182–191.
- Kleinridders, A.; Pothos, E.N. Impact of brain insulin signaling on dopamine function, food intake, reward, and emotional behavior. Curr. Nutr. Rep. 2019, 8, 83–91.
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 2016, 96, 1169–1209.
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534.
- Sallam, N.A.; Borgland, S.L. Insulin and endocannabinoids in the mesolimbic system. J. Neuroendocrinol. 2021, 33, e12965.
- Chen, W.; Cai, W.; Hoover, B.; Kahn, C.R. Insulin action in the brain: Cell types, circuits and diseases. Trends Neurosci. 2022, 45, 384–400.
- Ferrario, C.R.; Finnell, J.E. Beyond the hypothalamus: Roles for insulin as a regulator of neurotransmission, motivation, and feeding. Neuropsychopharmacology 2023, 48, 232–233.
- Cachope, R.; Mateo, Y.; Mathur, B.N.; Irving, J.; Wang, H.L.; Morales, M.; Lovinger, D.M.; Cheer, J.F. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Rep. 2012, 2, 33–41.
- Threlfell, S.; Lalic, T.; Platt, N.J.; Jennings, K.A.; Deisseroth, K.; Cragg, S.J. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75, 58–64.
- Liu, C.; Cai, X.; Ritzau-Jost, A.; Kramer, P.; Li, Y.; Khaliq, Z.M.; Hallermann, S.; Kaeser, P.S. An action potential intiation mechanism in distal axons for the control of dopamine release. Science 2022, 375, 1378–1385.
- Kramer, P.F.; Brill-Weil, S.G.; Cummins, A.C.; Zhang, R.; Camacho-Hernandez, G.A.; Newman, A.H.; Eldridge, M.A.G.; Averbeck, B.B.; Khaliq, Z.M. Synaptic-like axo-axonal transmission from striatal cholinergic interneurons onto dopaminergic fibers. Neuron 2022, 18, 2949–2960.
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bein, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 2018, 174, 1015–1030.
- Sanchez, G.; Rodriguez, M.J.; Pomata, P.; Rela, L.; Murer, M.G. Reduction of an afterhyperpolarization current increases excitability in striatal cholinergic interneurons in rat parkinsonism. J. Neurosci. 2011, 31, 6553–6564.
- Bennett, B.D.; Callaway, J.C.; Wilson, C.J. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J. Neurosci. 2000, 20, 8493–8503.
- Patel, J.C.; Rice, M.E. Monitoring axonal and somatodendritic dopamine release using fast-scan cyclic voltammetry in brain slices. Methods Mol. Biol. 2013, 96, 243–273.
- Patel, J.C. Voltammetry: Electrochemical detection of neurotransmitters in the brain. In Encyclopedia of Life Sciences (eLS); John Wiley & Sons, Ltd.: Chichester, UK, 2016.
- Rice, M.E.; Cragg, S.J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004, 7, 583–584.
- Patel, J.C.; Rossignol, E.; Rice, M.E.; Machold, R.P. Opposing regulation of striatal dopamine release and exploratory motor behavior by forebrain and brainstem cholinergic inputs. Nat. Commun. 2012, 3, 1172.
- Rice, M.E.; Patel, J.C.; Cragg, S.J. Dopamine release in the basal ganglia. Neuroscience 2011, 198, 112–137.
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016, 6, 123–148.
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. In Endotext ; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK378978/ (accessed on 24 May 2022).
- Nirenberg, M.J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. 1996, 16, 436–447.
- Nirenberg, M.J.; Chan, J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. Immunogold localization of the dopamine transporter: An ultrastructural study of the rat ventral tegmental area. J. Neurosci. 1997, 17, 5255–5262.
- Hersch, S.M.; Yi, H.; Heilman, C.J.; Edwards, R.H.; Levey, A.I. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J. Comp. Neurol. 1997, 388, 211–227.
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. NPJ Parkinsons Dis. 2021, 7, 22.
- Ryan, R.M.; Ingram, S.L.; Scimemi, A. Regulation of Glutamate, GABA and Dopamine Transporter Uptake, Surface Mobility and Expression. Front. Cell. Neurosci. 2021, 15, 670346.
- Jones, S.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034.
- Figlewicz, D.P.; Szot, P.; Chavez, M.; Woods, S.C.; Veith, R.C. Intraventricular insulin increases dopamine transporter mRNA in rat VTA/substantia nigra. Brain Res. 1994, 644, 331–334.
- Carvelli, L.; Moron, J.A.; Kahlig, K.M.; Ferrer, J.V.; Sen, N.; Lechleiter, J.D.; Leeb-Lundberg, L.M.F.; Merrill, G.; Lafer, E.M.; Ballou, L.M.; et al. PI3-kinase regulation of dopamine uptake. J. Neurochem. 2002, 81, 859–869.
- Garcia, B.G.; Wei, Y.; Moron, J.A.; Lin, R.Z.; Javitch, J.A.; Galli, A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol. Pharmacol. 2005, 68, 102–109.
- Fagan, R.R.; Kearney, P.J.; Melikian, H.E. In Situ regulated dopamine transporter trafficking: There’s no place like home. Neurochem. Res. 2020, 45, 1335–1343.
- Schoffelmeer, A.N.; Drukarch, B.; De Vries, T.J.; Hoenboom, F.; Schetters, D.; Pattij, T. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J. Neurosci. 2011, 31, 1284–1291.
- Jones, K.T.; Woods, C.; Zhen, J.; Antonio, T.; Carr, K.D.; Reith, M.E.A. Effects of diet and insulin on dopamine-transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem. 2017, 140, 728–740.
- Patel, J.C.; Stouffer, M.A.; Mancini, M.; Nicholson, C.; Carr, K.D.; Rice, M.E. Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur. J. Neurosci. 2019, 49, 794–804.
- Wightman, R.M.; Amatore, C.; Engstrom, R.C.; Hale, P.D.; Kristensen, E.W.; Kuhr, W.G.; May, L.J. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 1988, 25, 513–523.
- Nicholson, C. Interaction between diffusion and Michaelis-Menten uptake of dopamine after iontophoresis in striatum. Biophys. J. 1995, 68, 1699–1715.
- Fordahl, S.C.; Jones, S.R. High-fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chem. Neurosci. 2017, 8, 290–299.
- Marshall, J.F.; O’Dell, S.J.; Navarrete, R.; Rosenstein, A.J. Dopamine high-affinity transport site topography in rat brain: Major differences between dorsal and ventral striatum. Neuroscience 1990, 37, 11–21.
- Ciliax, B.J.; Heilman, C.; Demchyshyn, L.L.; Pristupa, Z.B.; Ince, E.; Hersch, S.M.; Niznik, H.B.; Levey, A.I. The dopamine transporter: Immunochemical characterization and localization in brain. J. Neurosci. 1995, 15, 1714–1723.
- Gonzalez-Hernandez, T.; Barroso-Chinea, P.; De La Cruz Muros, I.; Del Mar Perez-Delgado, M.; Rodriguez, M. Expression of dopamine and vesicular monoamine transporters and differential vulnerability of mesostriatal dopaminergic neurons. J. Comp. Neurol. 2004, 479, 198–215.
- Condon, M.D.; Platt, N.J.; Zhang, Y.F.; Roberts, B.M.; Clements, M.A.; Vietti-Michelina, S.; Tseu, M.Y.; Brimblecombe, K.R.; Threlfell, S.; Mann, E.O.; et al. Plasticity in striatal dopamine release is governed by release-independent depression and the dopamine transporter. Nat. Commun. 2019, 10, 1–15.
- Orosco, M.; Rouch, C.; Gripois, D.; Blouquit, M.F.; Roffi, J.; Jacquot, C.; Cohen, Y. Striatal dopamine metabolism is differentially affected by insulin according to the genotype in Zucker rats: A microdialysis study. Psychoneuroendocrinology 1992, 17, 443–452.
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468.
- Gerfen, C.R.; Engber, T.M.; Mahan, L.C.; Susel, Z.; Chase, T.N.; Monsma, F.J., Jr.; Sibley, D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990, 250, 1429–1432.
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466.
- Fetterly, T.L.; Oginsky, M.F.; Nieto, A.M.; Alonso-Caraballo, Y.; Santana-Rodriguez, Z.; Ferrario, C.R. Insulin bidirectionally alters NAc glutamatergic transmission: Interactions between insulin receptor activation, endogenous opioids, and glutamate release. J. Neurosci. 2021, 41, 2360–2372.
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389.
- Hasel, P.; Liddelow, S.A. Astrocytes. Curr. Biol. 2021, 31, R326–R327.
- Verkhratsky, A.; Parpura, V.; Li, B.; Scuderi, C. Astrocytes: The housekeepers and guardians of the CNS. Adv. Neurobiol. 2021, 26, 21–53.
- Goubard, V.; Fino, E.; Venance, L. Contribution of astrocytic glutamate and GABA uptake to corticostriatal information processing. J. Physiol. 2011, 589, 2301–2319.
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter release from astrocytes: Functional, developmental, and pathological implications in the brain. Front. Neurosci. 2015, 9, 499.
- Liu, J.; Feng, X.; Wang, Y.; Xia, X.; Zheng, J.C. Astrocytes: GABAceptive and GABAergic cells in the brain. Front. Cell. Neurosci. 2022, 16, 892497.
- Sa, M.; Park, M.G.; Lee, C.J. Role of hypothalamic reactive astrocytes in diet-induced obesity. Mol. Cell 2022, 45, 65–75.
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 2014, 63, 2232–2243.
- García-Cáceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.X.; et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 2016, 166, 867–880.
- González-García, I.; Gruber, T.; García-Cáceres, C. Insulin action on astrocytes: From energy homeostasis to behaviour. J. Neuroendocrinol. 2021, 33, e12953.
- Heni, M.; Hennige, A.M.; Peter, A.; Siegel-Axel, D.; Ordelheide, A.M.; Krebs, N.; Machicao, F.; Fritsche, A.; Häring, H.U.; Staiger, H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE 2011, 6, e21594.
- Cai, W.; Xue, C.; Sakaguchi, M.; Konishi, M.; Shirazian, A.; Ferris, H.A.; Li, M.E.; Yu, R.; Kleinridders, A.; Pothos, E.N.; et al. Insulin regulates astrocyte gliotransmission and modulates behavior. J. Clin. Investig. 2018, 128, 2914–2926.




