Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tom G Moreels | -- | 2591 | 2023-06-27 12:54:38 | | | |
| 2 | Rita Xu | Meta information modification | 2591 | 2023-06-28 04:06:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Monino, L.; Moreels, T.G. Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract. Encyclopedia. Available online: https://encyclopedia.pub/entry/46119 (accessed on 08 February 2026).
Monino L, Moreels TG. Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract. Encyclopedia. Available at: https://encyclopedia.pub/entry/46119. Accessed February 08, 2026.
Monino, Laurent, Tom G. Moreels. "Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract" Encyclopedia, https://encyclopedia.pub/entry/46119 (accessed February 08, 2026).
Monino, L., & Moreels, T.G. (2023, June 27). Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract. In Encyclopedia. https://encyclopedia.pub/entry/46119
Monino, Laurent and Tom G. Moreels. "Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract." Encyclopedia. Web. 27 June, 2023.
Copy Citation
Anastomotic leaks after gastrointestinal surgery have an important impact on surgical outcomes because of the high morbidity and mortality rates. Multiple treatment options exist requiring an individualized patient-tailored treatment plan after multidisciplinary discussion. Endoscopic vacuum therapy (EVT) is a novel treatment option that is nowadays recognized as an effective and useful endoscopic approach to treat leaks or perforations in both the upper and lower gastrointestinal tract.
upper gastrointestinal tract
anastomotic leak
endoscopic vacuum therapy
1. Introduction
Upper gastrointestinal tract surgery for both benign and malignant indications is prone to postoperative adverse events, with anastomotic leakage being one of the most challenging to treat. The risk and the location of the anastomotic leak depends on the type of surgical intervention and different classifications of anastomotic leaks exist [1][2]. The site of the leak and the extent of the corresponding extraluminal collection depends on the type of surgical intervention [2]. Despite new developments such as minimally invasive and robotic-assisted surgical techniques, anastomotic leak remains a frequent adverse event, occurring in up to one out of three patients and usually appearing in the early postoperative period [3][4][5]. Leaks may vary from a minor anastomotic fistula to complete dehiscence of the anastomosis, greatly impacting the surgical outcome, with high morbidity and mortality up to 25% [5][6][7]. Apart from the major clinical implications, postoperative anastomotic leaks after oesophageal surgery also have an important economic impact, nearly doubling the amount of the admission cost compared to non-complicated surgery [8]. A patient-tailored approach is required, and different therapeutic options are currently available, ranging from redo surgery, interventional radiology and endoscopy, often with a combination of techniques, depending on local availability and expertise, but also on size and type of the defect [9][10]. None of these rescue techniques is currently superior over the other ones. Therefore, possible therapeutic options should be discussed on a multidisciplinary base with the surgeons, radiologists and endoscopists to optimize the treatment plan [11]. The patient should be informed about the duration of the treatment, the potential risks and the expected outcome.
With the advent of new techniques and therapeutic options, endoscopy has become an important player in the treatment of anastomotic leaks in the upper gastrointestinal tract [12]. The endoscopic approach has been reviewed recently and encompasses internal drainage techniques of extramural collections using double-pigtail stents and closure techniques of the anastomotic defect using covered metallic stents, through-the-scope or over-the-scope clips, and endoluminal suturing [9][12][13][14]. A newly adopted option is endoscopic vacuum therapy (EVT), which has been shown to be very effective in draining extramural collections, reducing their size to even complete closure of the anastomotic defect, or improving the inflammatory tissue environment before redo surgery [15][16][17][18]. Prospective randomized comparative studies are currently missing due to the often life-threatening condition of the patients with postoperative leaks, requiring an individualized patient-tailored approach. Research data are therefore based on retrospective case series, with a possible selection bias when trying to compare the efficacy and safety of the different available techniques. EVT is currently accepted as a valuable endoscopic treatment option of large-sized anastomotic leaks in both the upper and the lower gastrointestinal tract and appears to be more effective and less burdened by adverse events as compared to metallic stenting according to a recent meta-analysis published in this journal [19][20]. It was also shown to be useful for the treatment of duodenal perforations and defects after biliopancreatic surgery [12].
2. Difficulties of Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract
Despite the fact that EVT is nowadays a standardized procedure, it remains an enterprise prone to several hurdles and difficulties. They can be encountered in the pre-, intra- and post-procedure period, and also during the removal of the sponge and drainage tube.
2.1. Pre-Procedure Difficulties
Not all oesophageal perforations can be treated using EVT [21]. The technique relies on the collapse of the extramural collection under negative pressure. The presence of an oesophageal fistula to the respiratory system does not permit the necessary build-up of negative pressure, thus rendering EVT unsuccessful. Therefore, EVT should not be used to treat broncho-oesophageal fistulas, although there are a few case reports of successful EVT treatment [22]. Secondly, since the device stimulates local tissue perfusion, it is considered contra-indicated in a malignant environment because of the risk of tumour growth induction. Finally, complete anastomotic dehiscence due to ischemic conduit necrosis necessitates redo surgery, and EVT should not be attempted in these particular situations (beyond EVT). Pre-procedure evaluation with CT scan (with oral contrast) and upper gastrointestinal endoscopy is mandatory to correctly evaluate the indication for EVT and to facilitate a patient-tailored multidisciplinary treatment plan (Figure 1).
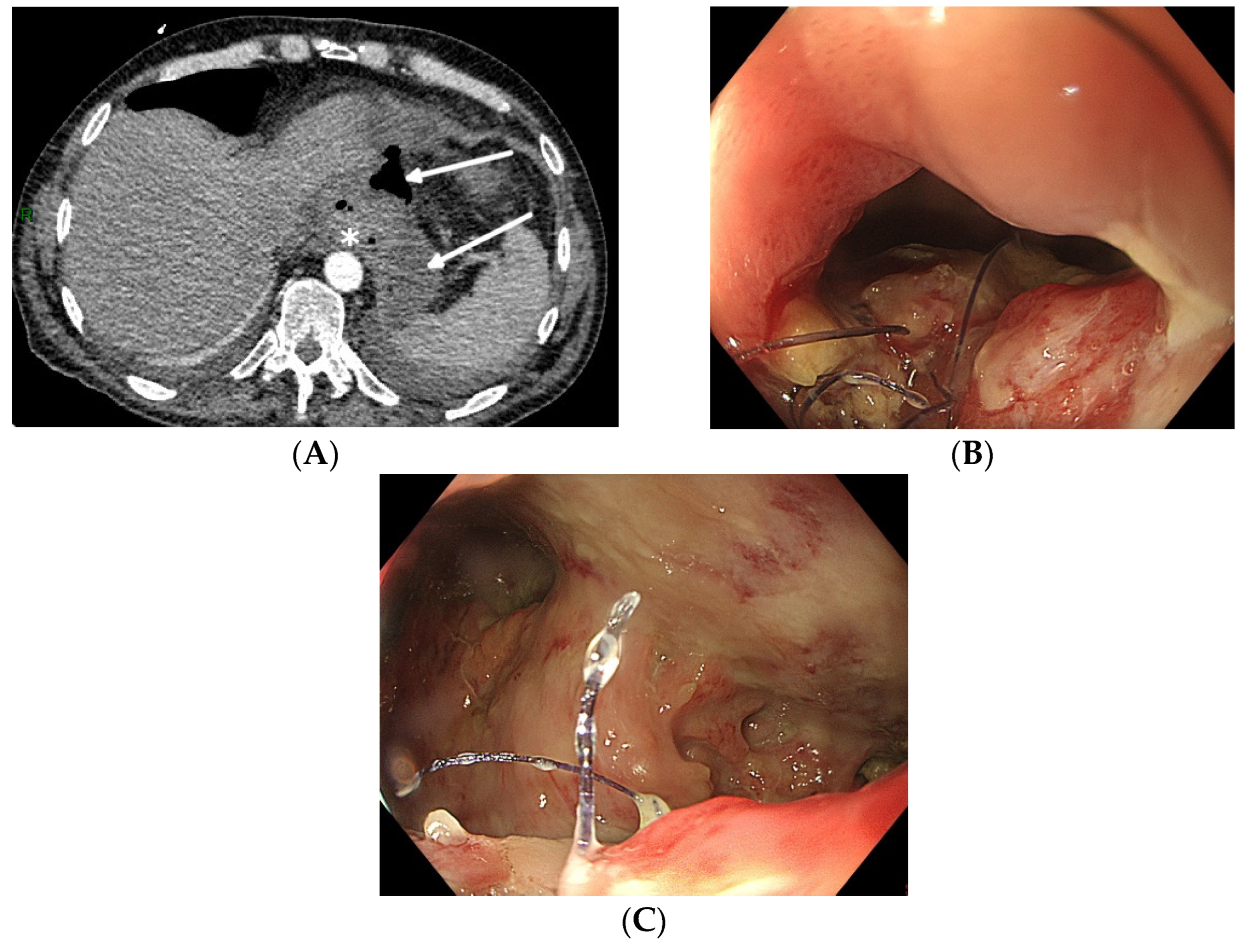
Figure 1. Total gastrectomy with an oesophagojejunal anastomosis complicated by an anastomotic leak. (A) Pre-procedure evaluation by CT scan (white arrows: hydroaeric collection; white star: anastomotic leak). (B) Pre-procedure endoscopic evaluation with inspection of the anastomotic leak. (C) Pre-procedure endoscopic evaluation of the extramural cavity.
Although ready-to-use sets are commercially available (Endo-Sponge, Eso-Sponge; B. Braun Melsungen AG; Melsungen, Germany), their availability is not widespread, and their financial cost is significant. However, since the design of the device is not very complicated, it can be self-assembled by attaching an open-pore drainage film or a size-cut polyurethane sponge to a nasogastric tube with multiple side-holes around the distal tip [23][24]. It can be introduced into the cavity through the commercially available overtube, or it can be endoscopically manoeuvred using rat tooth forceps or a polypectomy snare, taking care not to damage the sponge during the introduction. In the absence of an electronic vacuum pump with alarm function, a vacuum redon drain or bottle can be used as a continuous vacuum source. However, neither the redon drain nor bottle allows researchers to pre-set the vacuum force precisely (high–medium–low), and regular evaluation of the vacuum force is mandatory when using these alternative vacuum sources. Whenever the vacuum suction force drops because the redon drain is filled with aspirated fluids and debris, it should be replaced immediately. The loss of continuous vacuum aspiration negatively impacts EVT efficacy.
2.2. Intra-Procedure Difficulties
When engaging into the EVT procedure of oesophageal anastomotic leaks, several difficulties can be encountered. Since this is a time-consuming procedure with multiple introductions of the endoscope, it is best performed under general anaesthesia with endotracheal intubation, to prevent secretions and debris from the collection entering the respiratory system and to improve working conditions for both the endoscopist as for the patient. Fluoroscopy may also help to evaluate the depth and the extent of the extramural collection at the beginning of the EVT treatment, without being mandatory for future sponge exchanges (Figure 2).
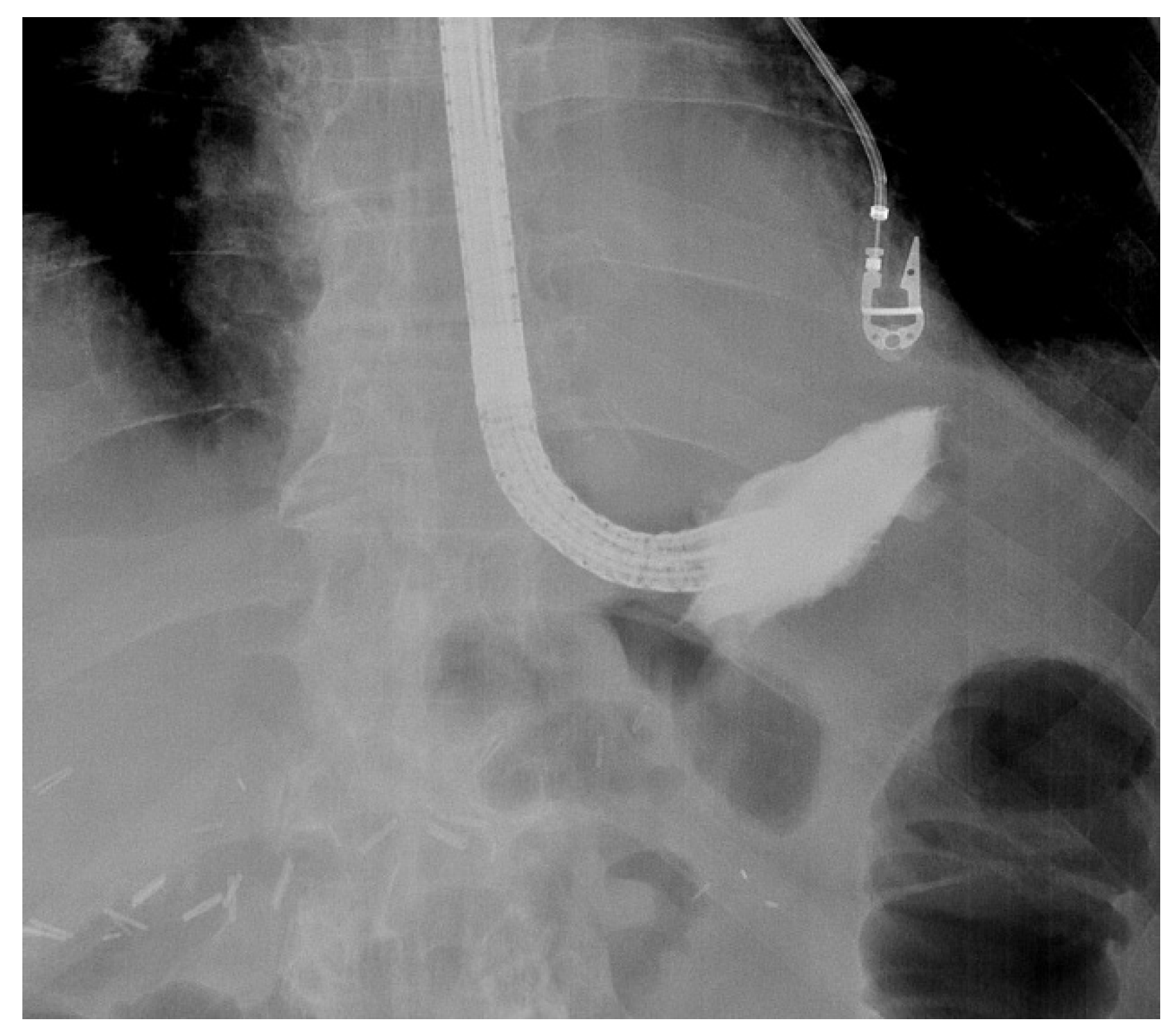
Figure 2. Fluoroscopic evaluation of the depth and the orientation of an extramural collection after endoscopic contrast injection in a patient with an anastomotic leak of an oesophagojejunal anastomosis.
The size of the anastomotic fistula may range from a millimetric orificium to a nearly complete dehiscence of the anastomosis [7]. A small-sized fistula can be closed using clips or stents or endoscopic suturing. However, external drainage of an extramural collection is mandatory when closing the fistula. Internal drainage using double pigtail stents can be an option, as well as EVT. However, when the fistula diameter does not allow the introduction of the overtube and the sponge, balloon dilatation of the fistula tract is possible. Additionally, the size of the sponge can be adapted to the small volume cavity by cutting it to a smaller and compatible size. Finally, positioning of the sponge into the oesophageal lumen covering the fistula orificium is another option to treat small-sized fistula [25]. The latter is usually a less efficacious solution, since the majority of the negative-pressure force will be lost in the lumen of the gastrointestinal tract, and other closure techniques with clips or endoscopic suturing may be more appropriate in case of small-sized fistula [9]. Intraluminal positioning of the sponge can also be applied at the end of successful EVT treatment with collapse of the extramural cavity to close the remaining small-sized fistula tract. The intraluminal sponge placement provokes stasis of saliva above the sponge. As already mentioned, the size of the sponge can be adapted to the volume of a small collection by cutting the sponge to its correct size. Large-sized fistulas and collections or near-complete anastomotic dehiscence may need the introduction of more than one sponge in order to optimize vacuum therapy and to promote the collapse of the collection (Figure 3).
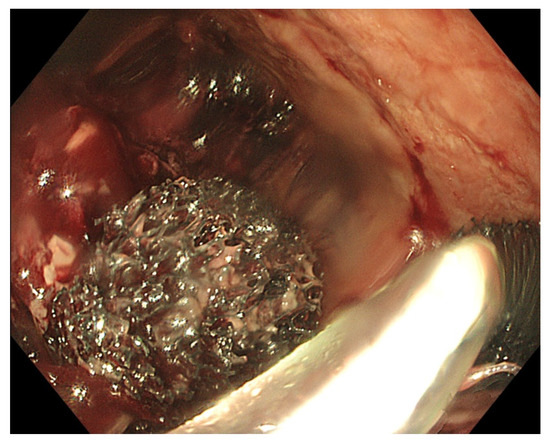
Figure 3. Placement of two sponges in a large-sized extramural collection (80 × 70 mm).
When opting for more than one EVT device, each device should be connected to its proper vacuum source to avoid the loss of aspiration through communicating vessels. In case of anastomotic dehiscence without extraluminal collection, the sponge should be positioned in the anastomosis maintaining a local negative pressure of −75 mm Hg to −125 mm Hg. However, this approach can induce an anastomotic fibrotic stricture as a result of successful EVT (Figure 4).
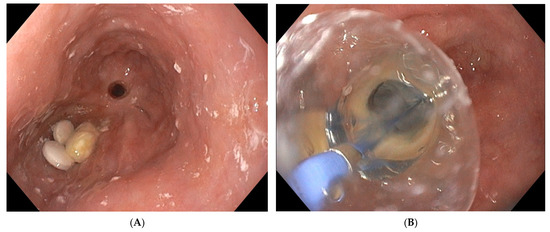
Figure 4. (A) Oesophageal stricture 6 months after intraluminal EVT treatment of partial anastomotic dehiscence. Notice the presence of medication tablets proximal to the post-EVT anastomotic stricture. (B) Endoscopic balloon dilatation of a post-EVT oesophageal stricture.
Large collections may contain necrotic debris, pus and fibrine. To avoid early saturation of the sponge with necrotic debris and a loss of negative-pressure force, the collection should be cleaned as much as possible before positioning the sponge. This can be performed via removal of the debris using rat tooth forceps, a polypectomy snare or mere rinsing and aspiration through the working channel of the endoscope. This cleaning procedure is indicated at every new sponge exchange.
Successful sponge positioning relies on the correct introduction of the overtube into the collection. This can be challenging when the access to the collection requires an angulation of the tip of the endoscope (Figure 5).
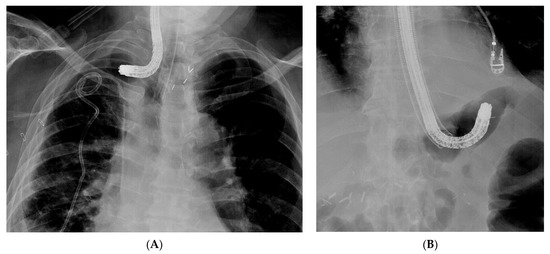
Figure 5. Difficult introduction of the overtube on the endosope into the collection. (A) Introduction of the endoscope into a collection, thus complicating an anastomotic oesophagogastric leak. The angulated tip of the endoscope does not allow advancement of the overtube into the collection. (B) Introduction of the overtube is not possible over the angulated endoscope tip in a patient with an anastomotic leak and extramural collection at the level of the oesophagojejunal anastomosis.
Moreover, introducing the sponge through the overtube comes with a lot of friction, and it increases the risk of dislocation of the overtube from the collection into the oesophageal lumen. To reduce the friction, it is advised to use a silicon spray to lubricate the inner lumen of the overtube and to apply lubrification gel onto the sponge to facilitate its introduction through the overtube. Endoscopic control of the correct position of the sponge into the collection is warranted, since dislocation of both the overtube and the sponge is possible during the introduction process. In case of incorrect positioning of the sponge after removal of the overtube, the sponge should be removed, and the procedure should be restarted. As an alternative, the sponge can be endoscopically manoeuvred from the oesophageal lumen into the collection using rat tooth forceps, taking care not to damage the sponge while trying to reposition it. Additionally, in case of a self-assembled device lacking the overtube, the sponge-loaded tube can be taken by rat tooth forceps or a polypectomy snare before it is introduced endoscopically through the mouth and taken down into the oesophagus and the collection [23]. Correct positioning of the sponge into the collection or intraluminally adjacent to the fistula is mandatory for successful EVT. Migration of the sponge will negatively affect EVT efficacy. Endoscopic control of the correct position is therefore mandatory at the beginning and at the end of each sponge exchange procedure.
Although the manufacturer’s product information defines the position of the sponge-loaded flexible tube in the mouth, it is much more comfortable for the patient to position it through the nose like a classical nasogastric tube. Transnasal position of the tube will also avoid occlusion of the tube (and thus a loss of negative pressure) by biting it. The tube can be redirected from the mouth to the nose using the rendezvous technique with a naso-oral catheter. Transnasal position does not prevent the removal of the tube and the soft sponge through the nose, but this should only be performed under general anaesthesia with endotracheal intubation, in order to prevent aspiration of debris adherent to the sponge and to optimize the patient’s clinical comfort. The majority of patients undergoing EVT in the upper gastrointestinal tract are hospitalized until the end of the endoscopic treatment, but only a minority needs admission to the Intensive Care Unit.
2.3. Post-Procedure Difficulties
After exteriorisation of the tube through the nose, gentle traction should be applied before fixation of the tube, in order to maintain the sponge in the correct position and to avoid distal migration of the sponge. As mentioned before, endoscopic control of the correct final sponge position is warranted.
Connecting the vacuum source to the aspiration tube requires adjusted connectors depending on the type of vacuum source. As for the EVT device, a compatible electronic vacuum pump is not always available, and it can be replaced by a surgical vacuum redon drain or a vacuum bottle. These non-electronic vacuum sources usually allow high–medium–low aspiration force through a physical switch, in contrast to the electronic pump with an adjustable negative force up to −200 mm Hg. The use of a vacuum redon drain or bottle requires frequent evaluation of the negative pressure since there is no electronic alarm when the negative pressure drops too low. When the redon contains too much aspiration fluids from the collection, it should be replaced immediately in order to maintain effective negative pressure. Leaks at the connectors should also be looked for in case of a loss of negative pressure.
As mentioned before, EVT requires multiple replacements of the device in order to maintain a continuous negative pressure at the level of the anastomotic leak. Therefore, the sponge should be replaced every 3 to 4 days. A delay in the replacement of the sponge may, on the one hand, lead to a loss of negative pressure and, on the other hand, to gradual tissue ingrowth into the open pores of the sponge. This may render sponge removal more difficult, introducing the risk of sponge rupture [26]. Since multiple sponge replacements are required (usually during a procedure under general anaesthesia), the necessary endoscopy time slots in the upcoming weeks should be reserved once EVT is initiated and the first sponge is put in place. Reserved time slots every Monday and Thursday or every Tuesday and Friday are very practical, in order to avoid scheduling replacements during the weekends.
Transnasal removal of the sponge is feasible and safe, but should only be performed under general anaesthesia with endotracheal intubation to avoid aspiration of secretions, blood and debris in the respiratory system. Endoscopic evaluation of the correct position of the sponge is important before its removal to assure efficient EVT. It might be necessary to dislodge the sponge from the granulating tissue using the endoscope or rat tooth forceps to facilitate atraumatic dissection and removal. Endoscopic inspection of the residual cavity is not only mandatory to evaluate the reduction in its volume and the aspect of the granulation tissue, but also to exclude and remove retained fragments of the sponge, if any (Figure 6). Intracavitary bleeding may occur after sponge removal. It is usually diffuse oozing bleeding and represents a sign of favourable tissue healing (Figure 6). It generally stops after endoscopic rinsing and cleaning of the cavity. However, it is advised to stop anticoagulant therapy before removal of the sponge [21].
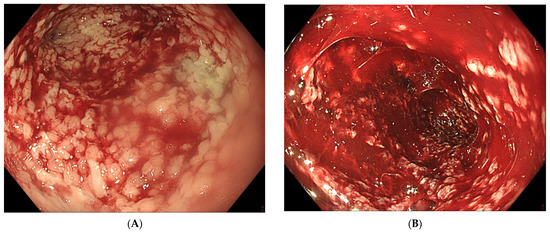
Figure 6. (A) Clean granular aspect of healing tissue in an extraluminal cavity after removal of the EVT sponge. (B) Diffuse intracavity bleeding after removal of the EVT sponge.
References
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; D’journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294.
- Ross, S.L.; Veluswamy, B.; Craig, E.V.; Miller, F.H.; Horowitz, J.M.; Kelahan, L.C. Optimizing detection of postoperative leaks on upper gastrointestinal fluoroscopy: A step-by-step guide. Abdom. Imaging 2021, 46, 3019–3032.
- Vetter, D.; Gutschow, C.A. Strategies to prevent anastomotic leakage after esophagectomy and gastric conduit reconstruction. Langenbeck’s Arch. Surg. 2020, 405, 1069–1077.
- Mils, K.; Miró, M.; Farran, L.; Videla, S.; Alba, E.; Estremiana, F.; Bettonica, C.; Aranda, H. A pilot randomized controlled trial on the utility of gastric conditioning in the prevention of esophagogastric anastomotic leak after Ivor Lewis esophagectomy. The APIL_2013 Trial. Int. J. Surg. 2022, 106, 106921.
- Baiocchi, G.L.; Giacopuzzi, S.; Vittimberga, G.; De Pascale, S.; Pastorelli, E.; Gelmini, R.; Viganò, J.; Graziosi, L.; Vagliasindi, A.; Rosa, F.; et al. Clinical outcomes of patients with complicated post-operative course after gastrectomy for cancer: A GIRCG study using the GASTRODATA registry. Updat. Surg. 2023, 75, 419–427.
- Ubels, S.; Verstegen, M.; Klarenbeek, B.; Bouwense, S.; Berge Henegouwen, M.; Daams, F.; van Det, M.J.; Griffiths, E.A.; Haveman, J.W.; Heisterkamp, J.; et al. Severity of oEsophageal Anastomotic Leak in patients after oesophagectomy: The SEAL score. Br. J. Surg. 2022, 109, 864–871.
- Bachmann, J.; Feith, M.; Schlag, C.; Abdelhafez, M.; Martignoni, M.E.; Friess, H. Anastomotic leakage following resection of the esophagus—Introduction of an endoscopic grading system. World J. Surg. Oncol. 2022, 20, 104.
- Browning, A.F.; Chong, L.; Read, M.; Hii, M.W. Economic burden of complications and readmission following oesophageal cancer surgery. ANZ J. Surg. 2022, 92, 2901–2906.
- Chan, S.M.; Auyeung, K.K.Y.; Lam, S.F.; Chiu, P.W.Y.; Teoh, A.Y.B. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig. Endosc. 2022, 34, 43–62.
- Ubels, S.; Lubbers, M.; Verstegen, M.H.P.; Bouwense, S.A.W.; van Daele, E.; Ferri, L.; Gisbertz, S.S.; Griffiths, E.A.; Grimminger, P.; Hanna, G.; et al. Treatment of anastomotic leak after esophagectomy: Insights of an international case vignette survey and expert discussions. Dis. Esophagus 2022, 35, doac020.
- Stavropoulos, S.N.; Modayil, R.; Friedel, D. Closing Perforations and Postperforation Management in Endoscopy. Gastrointest. Endosc. Clin. N. Am. 2015, 25, 29–45.
- Binda, C.; Jung, C.F.M.; Fabbri, S.; Giuffrida, P.; Sbrancia, M.; Coluccio, C.; Gibiino, G.; Fabbri, C. Endoscopic Management of Postoperative Esophageal and Upper GI Defects—A Narrative Review. Medicina 2023, 59, 136.
- Goenka, M.K.; Goenka, U. Endotherapy of leaks and fistula. World J. Gastrointest. Endosc. 2015, 7, 702.
- Cereatti, F.; Grassia, R.; Drago, A.; Conti, C.B.; Donatelli, G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J. Gastroenterol. 2020, 26, 4198–4217.
- Jung, D.H.; Yun, H.-R.; Lee, S.J.; Kim, N.W.; Huh, C.W. Endoscopic Vacuum Therapy in Patients with Transmural Defects of the Upper Gastrointestinal Tract: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 2346.
- Junior, E.S.D.M.; De Moura, D.T.H.; Ribeiro, I.B.; Hathorn, K.E.; Farias, G.F.A.; Turiani, C.V.; Medeiros, F.S.; Bernardo, W.M.; De Moura, E.G.H. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig. Endosc. 2021, 33, 892–902.
- Scognamiglio, P.; Reeh, M.; Melling, N.; Kantowski, M.; Eichelmann, A.-K.; Chon, S.-H.; El-Sourani, N.; Höller, A.; Izbicki, J.R.; Tachezy, M. Management of intra-thoracic anastomotic leakages after esophagectomy: Updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg. 2022, 22, 309.
- Intriago, J.M.V.; de Moura, D.T.H.; Junior, E.S.D.M.; Proença, I.M.; Ribeiro, I.B.; Sánchez-Luna, S.A.; Bernardo, W.M.; de Moura, E.G.H. Endoscopic Vacuum Therapy (EVT) for the Treatment of Post-Bariatric Surgery Leaks and Fistulas: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 3435–3451.
- Tachezy, M.; Chon, S.-H.; Rieck, I.; Kantowski, M.; Christ, H.; Karstens, K.; Gebauer, F.; Goeser, T.; Rösch, T.; Izbicki, J.R.; et al. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): Study protocol for a prospective randomized phase 2 trial. Trials 2021, 22, 377.
- Mandarino, F.V.; Barchi, A.; D’amico, F.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Rosati, R.; Fiorino, G.; Bemelman, W.A.; et al. Endoscopic Vacuum Therapy (EVT) versus Self-Expandable Metal Stent (SEMS) for Anastomotic Leaks after Upper Gastrointestinal Surgery: Systematic Review and Meta-Analysis. Life 2023, 13, 287.
- Gutschow, C.A.; Schlag, C.; Vetter, D. Endoscopic vacuum therapy in the upper gastrointestinal tract: When and how to use it. Langenbeck’s Arch. Surg. 2022, 407, 957–964.
- Lee, H.J.; Lee, H. Endoscopic Vacuum-assisted Closure With Sponge for Esophagotracheal Fistula After Esophagectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2015, 25, e76–e77.
- Leeds, S.G.; Mencio, M.; Ontiveros, E.; Ward, M.A. Endoluminal Vacuum Therapy: How I Do It. J. Gastrointest. Surg. 2019, 23, 1037–1043.
- de Moura, D.T.H.; Hirsch, B.S.; McCarty, T.R.; dos Santos, M.E.L.; Guedes, H.G.; Gomes, G.F.; de Medeiros, F.S.; de Moura, E.G.H. Homemade endoscopic vacuum therapy device for the management of transmural gastrointestinal defects. Dig. Endosc. 2023; online ahead of print.
- Loske, G.; Müller, C.T. Tips and tricks for endoscopic negative pressure therapy. Der Chir. 2019, 90 (Suppl. S1), 7–14.
- Loske, G.; Schorsch, T.; Müller, C. Endoscopic vacuum sponge therapy for esophageal defects. Surg. Endosc. 2010, 24, 2531–2535.
More
Information
Subjects:
Gastroenterology & Hepatology; Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
736
Revisions:
2 times
(View History)
Update Date:
28 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




