Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Tom G Moreels.
Anastomotic leaks after gastrointestinal surgery have an important impact on surgical outcomes because of the high morbidity and mortality rates. Multiple treatment options exist requiring an individualized patient-tailored treatment plan after multidisciplinary discussion. Endoscopic vacuum therapy (EVT) is a novel treatment option that is nowadays recognized as an effective and useful endoscopic approach to treat leaks or perforations in both the upper and lower gastrointestinal tract.
- upper gastrointestinal tract
- anastomotic leak
- endoscopic vacuum therapy
1. Introduction
Upper gastrointestinal tract surgery for both benign and malignant indications is prone to postoperative adverse events, with anastomotic leakage being one of the most challenging to treat. The risk and the location of the anastomotic leak depends on the type of surgical intervention and different classifications of anastomotic leaks exist [1,2][1][2]. The site of the leak and the extent of the corresponding extraluminal collection depends on the type of surgical intervention [2]. Despite new developments such as minimally invasive and robotic-assisted surgical techniques, anastomotic leak remains a frequent adverse event, occurring in up to one out of three patients and usually appearing in the early postoperative period [3,4,5][3][4][5]. Leaks may vary from a minor anastomotic fistula to complete dehiscence of the anastomosis, greatly impacting the surgical outcome, with high morbidity and mortality up to 25% [5,6,7][5][6][7]. Apart from the major clinical implications, postoperative anastomotic leaks after oesophageal surgery also have an important economic impact, nearly doubling the amount of the admission cost compared to non-complicated surgery [8]. A patient-tailored approach is required, and different therapeutic options are currently available, ranging from redo surgery, interventional radiology and endoscopy, often with a combination of techniques, depending on local availability and expertise, but also on size and type of the defect [9,10][9][10]. None of these rescue techniques is currently superior over the other ones. Therefore, possible therapeutic options should be discussed on a multidisciplinary base with the surgeons, radiologists and endoscopists to optimize the treatment plan [11]. The patient should be informed about the duration of the treatment, the potential risks and the expected outcome.
With the advent of new techniques and therapeutic options, endoscopy has become an important player in the treatment of anastomotic leaks in the upper gastrointestinal tract [12]. The endoscopic approach has been reviewed recently and encompasses internal drainage techniques of extramural collections using double-pigtail stents and closure techniques of the anastomotic defect using covered metallic stents, through-the-scope or over-the-scope clips, and endoluminal suturing [9,12,13,14][9][12][13][14]. A newly adopted option is endoscopic vacuum therapy (EVT), which has been shown to be very effective in draining extramural collections, reducing their size to even complete closure of the anastomotic defect, or improving the inflammatory tissue environment before redo surgery [15,16,17,18][15][16][17][18]. Prospective randomized comparative studies are currently missing due to the often life-threatening condition of the patients with postoperative leaks, requiring an individualized patient-tailored approach. Research data are therefore based on retrospective case series, with a possible selection bias when trying to compare the efficacy and safety of the different available techniques. EVT is currently accepted as a valuable endoscopic treatment option of large-sized anastomotic leaks in both the upper and the lower gastrointestinal tract and appears to be more effective and less burdened by adverse events as compared to metallic stenting according to a recent meta-analysis published in this journal [19,20][19][20]. It was also shown to be useful for the treatment of duodenal perforations and defects after biliopancreatic surgery [12].
2. Difficulties of Endoscopic Vacuum Therapy in the Upper Gastrointestinal Tract
Despite the fact that EVT is nowadays a standardized procedure, it remains an enterprise prone to several hurdles and difficulties. They can be encountered in the pre-, intra- and post-procedure period, and also during the removal of the sponge and drainage tube.2.1. Pre-Procedure Difficulties
Not all oesophageal perforations can be treated using EVT [77][21]. The technique relies on the collapse of the extramural collection under negative pressure. The presence of an oesophageal fistula to the respiratory system does not permit the necessary build-up of negative pressure, thus rendering EVT unsuccessful. Therefore, EVT should not be used to treat broncho-oesophageal fistulas, although there are a few case reports of successful EVT treatment [42][22]. Secondly, since the device stimulates local tissue perfusion, it is considered contra-indicated in a malignant environment because of the risk of tumour growth induction. Finally, complete anastomotic dehiscence due to ischemic conduit necrosis necessitates redo surgery, and EVT should not be attempted in these particular situations (beyond EVT). Pre-procedure evaluation with CT scan (with oral contrast) and upper gastrointestinal endoscopy is mandatory to correctly evaluate the indication for EVT and to facilitate a patient-tailored multidisciplinary treatment plan (Figure 1).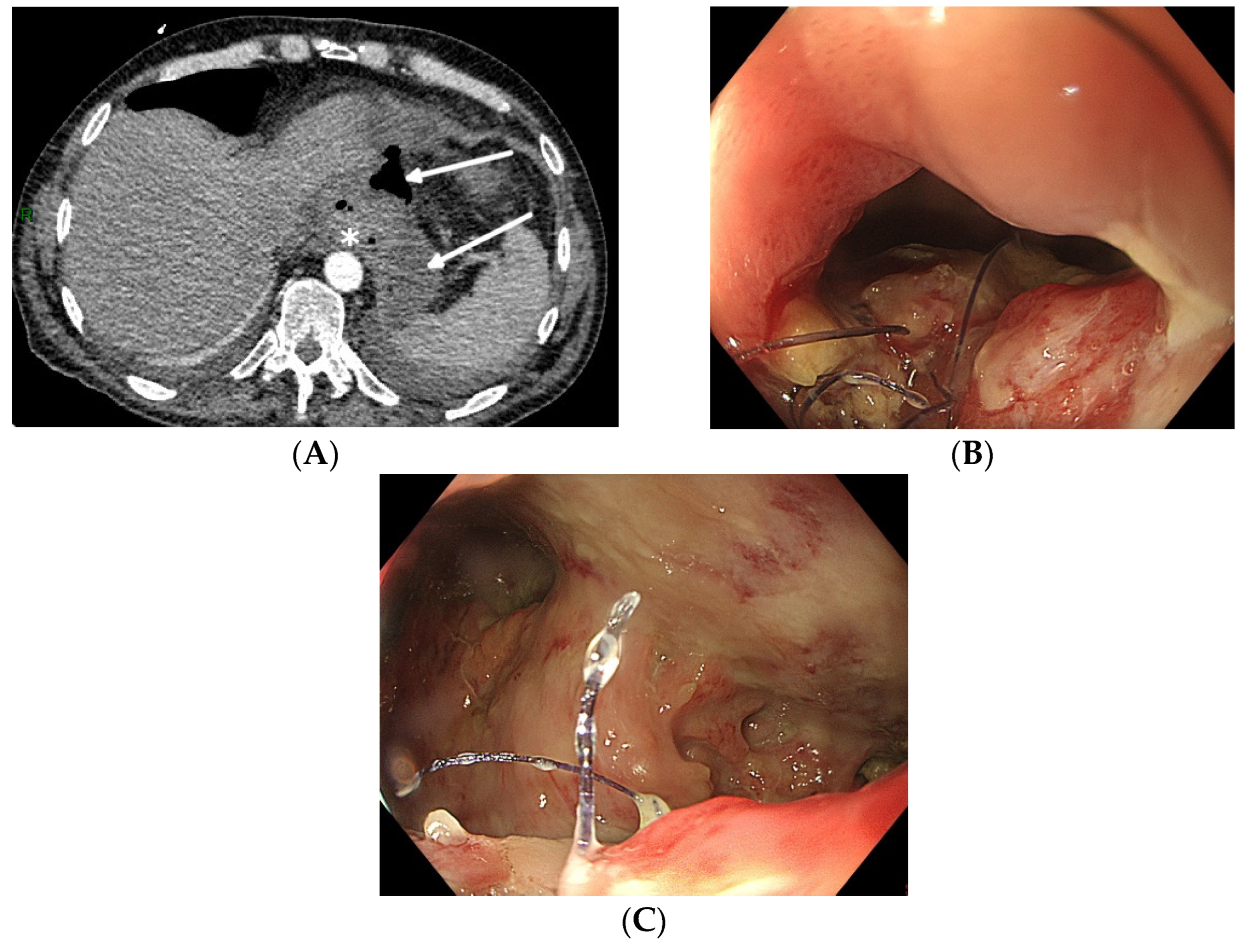
Figure 1. Total gastrectomy with an oesophagojejunal anastomosis complicated by an anastomotic leak. (A) Pre-procedure evaluation by CT scan (white arrows: hydroaeric collection; white star: anastomotic leak). (B) Pre-procedure endoscopic evaluation with inspection of the anastomotic leak. (C) Pre-procedure endoscopic evaluation of the extramural cavity.
2.2. Intra-Procedure Difficulties
When engaging into the EVT procedure of oesophageal anastomotic leaks, several difficulties can be encountered. Since this is a time-consuming procedure with multiple introductions of the endoscope, it is best performed under general anaesthesia with endotracheal intubation, to prevent secretions and debris from the collection entering the respiratory system and to improve working conditions for both the endoscopist as for the patient. Fluoroscopy may also help to evaluate the depth and the extent of the extramural collection at the beginning of the EVT treatment, without being mandatory for future sponge exchanges (Figure 2).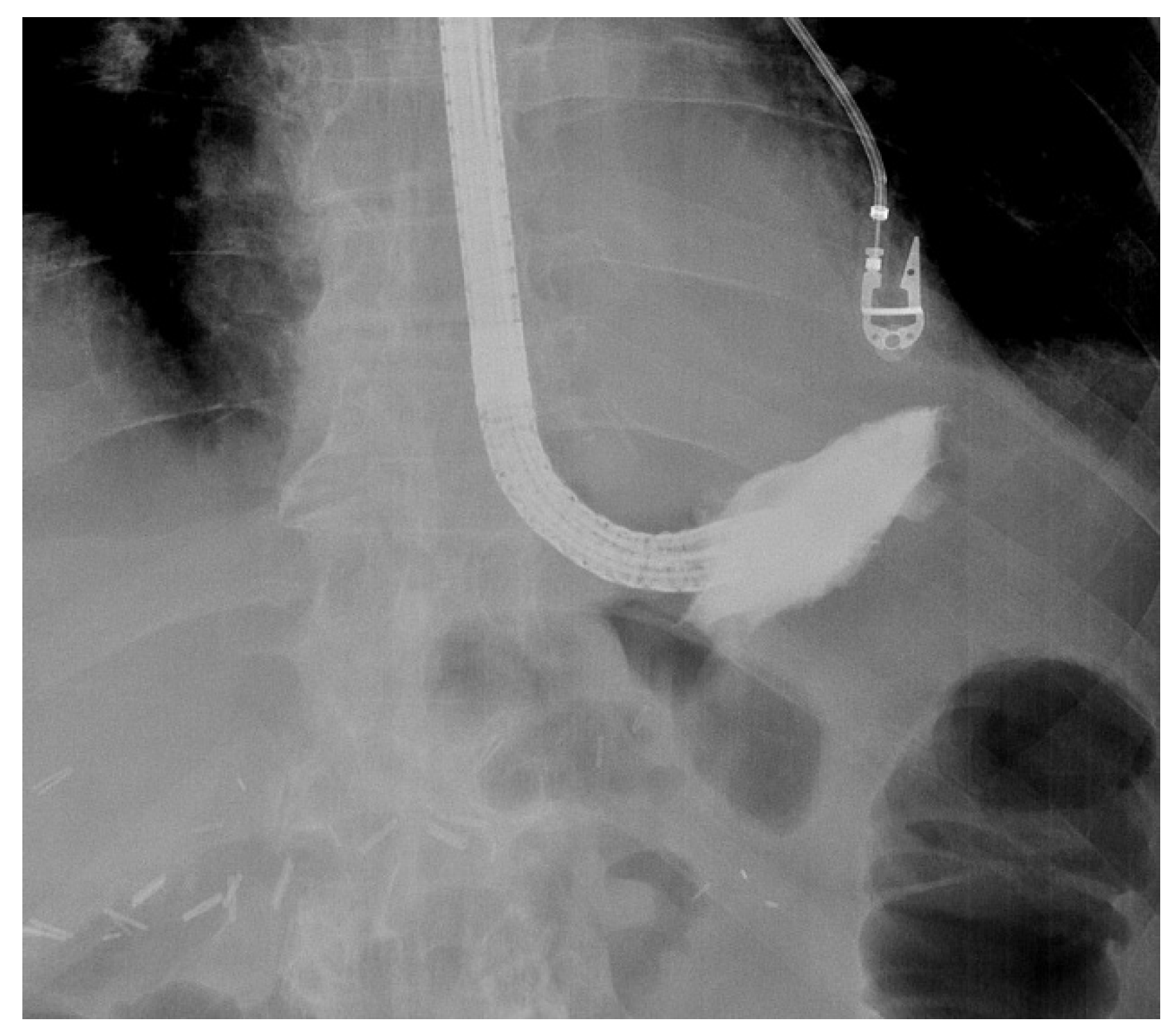
Figure 2. Fluoroscopic evaluation of the depth and the orientation of an extramural collection after endoscopic contrast injection in a patient with an anastomotic leak of an oesophagojejunal anastomosis.
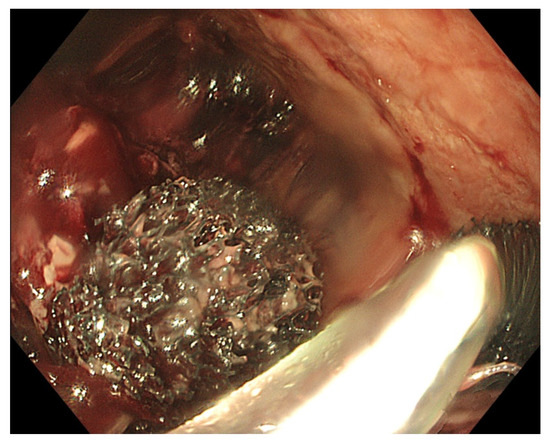
Figure 3. Placement of two sponges in a large-sized extramural collection (80 × 70 mm).
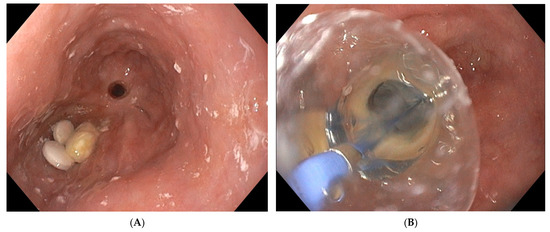
Figure 4. (A) Oesophageal stricture 6 months after intraluminal EVT treatment of partial anastomotic dehiscence. Notice the presence of medication tablets proximal to the post-EVT anastomotic stricture. (B) Endoscopic balloon dilatation of a post-EVT oesophageal stricture.
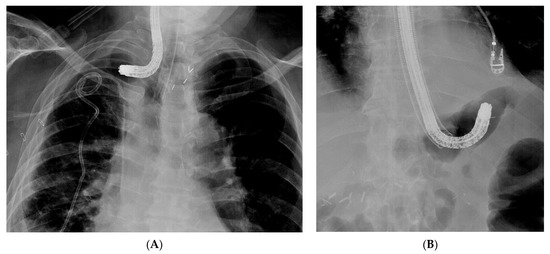
Figure 5. Difficult introduction of the overtube on the endosope into the collection. (A) Introduction of the endoscope into a collection, thus complicating an anastomotic oesophagogastric leak. The angulated tip of the endoscope does not allow advancement of the overtube into the collection. (B) Introduction of the overtube is not possible over the angulated endoscope tip in a patient with an anastomotic leak and extramural collection at the level of the oesophagojejunal anastomosis.
2.3. Post-Procedure Difficulties
After exteriorisation of the tube through the nose, gentle traction should be applied before fixation of the tube, in order to maintain the sponge in the correct position and to avoid distal migration of the sponge. As mentioned before, endoscopic control of the correct final sponge position is warranted. Connecting the vacuum source to the aspiration tube requires adjusted connectors depending on the type of vacuum source. As for the EVT device, a compatible electronic vacuum pump is not always available, and it can be replaced by a surgical vacuum redon drain or a vacuum bottle. These non-electronic vacuum sources usually allow high–medium–low aspiration force through a physical switch, in contrast to the electronic pump with an adjustable negative force up to −200 mm Hg. The use of a vacuum redon drain or bottle requires frequent evaluation of the negative pressure since there is no electronic alarm when the negative pressure drops too low. When the redon contains too much aspiration fluids from the collection, it should be replaced immediately in order to maintain effective negative pressure. Leaks at the connectors should also be looked for in case of a loss of negative pressure. As mentioned before, EVT requires multiple replacements of the device in order to maintain a continuous negative pressure at the level of the anastomotic leak. Therefore, the sponge should be replaced every 3 to 4 days. A delay in the replacement of the sponge may, on the one hand, lead to a loss of negative pressure and, on the other hand, to gradual tissue ingrowth into the open pores of the sponge. This may render sponge removal more difficult, introducing the risk of sponge rupture [29][26]. Since multiple sponge replacements are required (usually during a procedure under general anaesthesia), the necessary endoscopy time slots in the upcoming weeks should be reserved once EVT is initiated and the first sponge is put in place. Reserved time slots every Monday and Thursday or every Tuesday and Friday are very practical, in order to avoid scheduling replacements during the weekends. Transnasal removal of the sponge is feasible and safe, but should only be performed under general anaesthesia with endotracheal intubation to avoid aspiration of secretions, blood and debris in the respiratory system. Endoscopic evaluation of the correct position of the sponge is important before its removal to assure efficient EVT. It might be necessary to dislodge the sponge from the granulating tissue using the endoscope or rat tooth forceps to facilitate atraumatic dissection and removal. Endoscopic inspection of the residual cavity is not only mandatory to evaluate the reduction in its volume and the aspect of the granulation tissue, but also to exclude and remove retained fragments of the sponge, if any (Figure 6). Intracavitary bleeding may occur after sponge removal. It is usually diffuse oozing bleeding and represents a sign of favourable tissue healing (Figure 6). It generally stops after endoscopic rinsing and cleaning of the cavity. However, it is advised to stop anticoagulant therapy before removal of the sponge [77][21].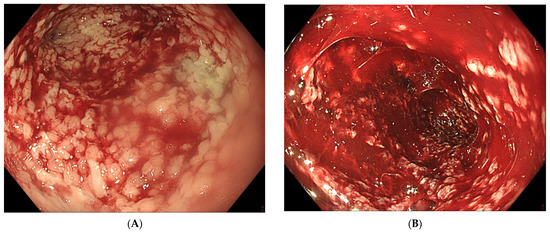
Figure 6. (A) Clean granular aspect of healing tissue in an extraluminal cavity after removal of the EVT sponge. (B) Diffuse intracavity bleeding after removal of the EVT sponge.
References
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; D’journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294.
- Ross, S.L.; Veluswamy, B.; Craig, E.V.; Miller, F.H.; Horowitz, J.M.; Kelahan, L.C. Optimizing detection of postoperative leaks on upper gastrointestinal fluoroscopy: A step-by-step guide. Abdom. Imaging 2021, 46, 3019–3032.
- Vetter, D.; Gutschow, C.A. Strategies to prevent anastomotic leakage after esophagectomy and gastric conduit reconstruction. Langenbeck’s Arch. Surg. 2020, 405, 1069–1077.
- Mils, K.; Miró, M.; Farran, L.; Videla, S.; Alba, E.; Estremiana, F.; Bettonica, C.; Aranda, H. A pilot randomized controlled trial on the utility of gastric conditioning in the prevention of esophagogastric anastomotic leak after Ivor Lewis esophagectomy. The APIL_2013 Trial. Int. J. Surg. 2022, 106, 106921.
- Baiocchi, G.L.; Giacopuzzi, S.; Vittimberga, G.; De Pascale, S.; Pastorelli, E.; Gelmini, R.; Viganò, J.; Graziosi, L.; Vagliasindi, A.; Rosa, F.; et al. Clinical outcomes of patients with complicated post-operative course after gastrectomy for cancer: A GIRCG study using the GASTRODATA registry. Updat. Surg. 2023, 75, 419–427.
- Ubels, S.; Verstegen, M.; Klarenbeek, B.; Bouwense, S.; Berge Henegouwen, M.; Daams, F.; van Det, M.J.; Griffiths, E.A.; Haveman, J.W.; Heisterkamp, J.; et al. Severity of oEsophageal Anastomotic Leak in patients after oesophagectomy: The SEAL score. Br. J. Surg. 2022, 109, 864–871.
- Bachmann, J.; Feith, M.; Schlag, C.; Abdelhafez, M.; Martignoni, M.E.; Friess, H. Anastomotic leakage following resection of the esophagus—Introduction of an endoscopic grading system. World J. Surg. Oncol. 2022, 20, 104.
- Browning, A.F.; Chong, L.; Read, M.; Hii, M.W. Economic burden of complications and readmission following oesophageal cancer surgery. ANZ J. Surg. 2022, 92, 2901–2906.
- Chan, S.M.; Auyeung, K.K.Y.; Lam, S.F.; Chiu, P.W.Y.; Teoh, A.Y.B. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig. Endosc. 2022, 34, 43–62.
- Ubels, S.; Lubbers, M.; Verstegen, M.H.P.; Bouwense, S.A.W.; van Daele, E.; Ferri, L.; Gisbertz, S.S.; Griffiths, E.A.; Grimminger, P.; Hanna, G.; et al. Treatment of anastomotic leak after esophagectomy: Insights of an international case vignette survey and expert discussions. Dis. Esophagus 2022, 35, doac020.
- Stavropoulos, S.N.; Modayil, R.; Friedel, D. Closing Perforations and Postperforation Management in Endoscopy. Gastrointest. Endosc. Clin. N. Am. 2015, 25, 29–45.
- Binda, C.; Jung, C.F.M.; Fabbri, S.; Giuffrida, P.; Sbrancia, M.; Coluccio, C.; Gibiino, G.; Fabbri, C. Endoscopic Management of Postoperative Esophageal and Upper GI Defects—A Narrative Review. Medicina 2023, 59, 136.
- Goenka, M.K.; Goenka, U. Endotherapy of leaks and fistula. World J. Gastrointest. Endosc. 2015, 7, 702.
- Cereatti, F.; Grassia, R.; Drago, A.; Conti, C.B.; Donatelli, G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J. Gastroenterol. 2020, 26, 4198–4217.
- Jung, D.H.; Yun, H.-R.; Lee, S.J.; Kim, N.W.; Huh, C.W. Endoscopic Vacuum Therapy in Patients with Transmural Defects of the Upper Gastrointestinal Tract: A Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 2346.
- Junior, E.S.D.M.; De Moura, D.T.H.; Ribeiro, I.B.; Hathorn, K.E.; Farias, G.F.A.; Turiani, C.V.; Medeiros, F.S.; Bernardo, W.M.; De Moura, E.G.H. Endoscopic vacuum therapy versus endoscopic stenting for upper gastrointestinal transmural defects: Systematic review and meta-analysis. Dig. Endosc. 2021, 33, 892–902.
- Scognamiglio, P.; Reeh, M.; Melling, N.; Kantowski, M.; Eichelmann, A.-K.; Chon, S.-H.; El-Sourani, N.; Höller, A.; Izbicki, J.R.; Tachezy, M. Management of intra-thoracic anastomotic leakages after esophagectomy: Updated systematic review and meta-analysis of endoscopic vacuum therapy versus stenting. BMC Surg. 2022, 22, 309.
- Intriago, J.M.V.; de Moura, D.T.H.; Junior, E.S.D.M.; Proença, I.M.; Ribeiro, I.B.; Sánchez-Luna, S.A.; Bernardo, W.M.; de Moura, E.G.H. Endoscopic Vacuum Therapy (EVT) for the Treatment of Post-Bariatric Surgery Leaks and Fistulas: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 3435–3451.
- Tachezy, M.; Chon, S.-H.; Rieck, I.; Kantowski, M.; Christ, H.; Karstens, K.; Gebauer, F.; Goeser, T.; Rösch, T.; Izbicki, J.R.; et al. Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): Study protocol for a prospective randomized phase 2 trial. Trials 2021, 22, 377.
- Mandarino, F.V.; Barchi, A.; D’amico, F.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Rosati, R.; Fiorino, G.; Bemelman, W.A.; et al. Endoscopic Vacuum Therapy (EVT) versus Self-Expandable Metal Stent (SEMS) for Anastomotic Leaks after Upper Gastrointestinal Surgery: Systematic Review and Meta-Analysis. Life 2023, 13, 287.
- Gutschow, C.A.; Schlag, C.; Vetter, D. Endoscopic vacuum therapy in the upper gastrointestinal tract: When and how to use it. Langenbeck’s Arch. Surg. 2022, 407, 957–964.
- Lee, H.J.; Lee, H. Endoscopic Vacuum-assisted Closure With Sponge for Esophagotracheal Fistula After Esophagectomy. Surg. Laparosc. Endosc. Percutaneous Tech. 2015, 25, e76–e77.
- Leeds, S.G.; Mencio, M.; Ontiveros, E.; Ward, M.A. Endoluminal Vacuum Therapy: How I Do It. J. Gastrointest. Surg. 2019, 23, 1037–1043.
- de Moura, D.T.H.; Hirsch, B.S.; McCarty, T.R.; dos Santos, M.E.L.; Guedes, H.G.; Gomes, G.F.; de Medeiros, F.S.; de Moura, E.G.H. Homemade endoscopic vacuum therapy device for the management of transmural gastrointestinal defects. Dig. Endosc. 2023; online ahead of print.
- Loske, G.; Müller, C.T. Tips and tricks for endoscopic negative pressure therapy. Der Chir. 2019, 90 (Suppl. S1), 7–14.
- Loske, G.; Schorsch, T.; Müller, C. Endoscopic vacuum sponge therapy for esophageal defects. Surg. Endosc. 2010, 24, 2531–2535.
More
