
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marzia Bruna Gariboldi | -- | 1903 | 2023-06-23 12:11:14 | | | |
| 2 | Lindsay Dong | Meta information modification | 1903 | 2023-06-25 03:48:03 | | |
Video Upload Options
Edible/medicinal mushrooms have been traditionally used in Asian countries either in the cuisine or as dietary supplements and nutraceuticals. Among the different pharmacological activities reported (antibacterial, anti-inflammatory, antioxidative, antiviral, immunomodulating, antidiabetic, etc.), edible/medicinal mushrooms have been shown to exert in vitro and in vivo anticancer effects on several kinds of tumors, including breast cancer.
1. Introduction
Breast cancer (BC) is among the major causes of cancer-related deaths worldwide, with 2.3 million new cases per year, according to the GLOBOCAN 2020 data [1]. Projections indicate that by 2030, the number of new cases diagnosed worldwide will reach 2.7 million, while the number of deaths will reach 0.9 million [2] (Global Cancer Observatory: Cancer Tomorrow, accessed on 29 April 2023. Available online: https://gco.iarc.fr/tomorrow). In addition, the incidence of breast cancer is expected to further increase, in particular in low- and medium-income countries, due to the effects of a westernized lifestyle, characterized by delayed pregnancies, reduced breastfeeding, low age at menarche, lack of physical activity, and poor diet [3].
2. Bioactive Compounds in Medicinal Mushrooms and Their Mechanisms of Action
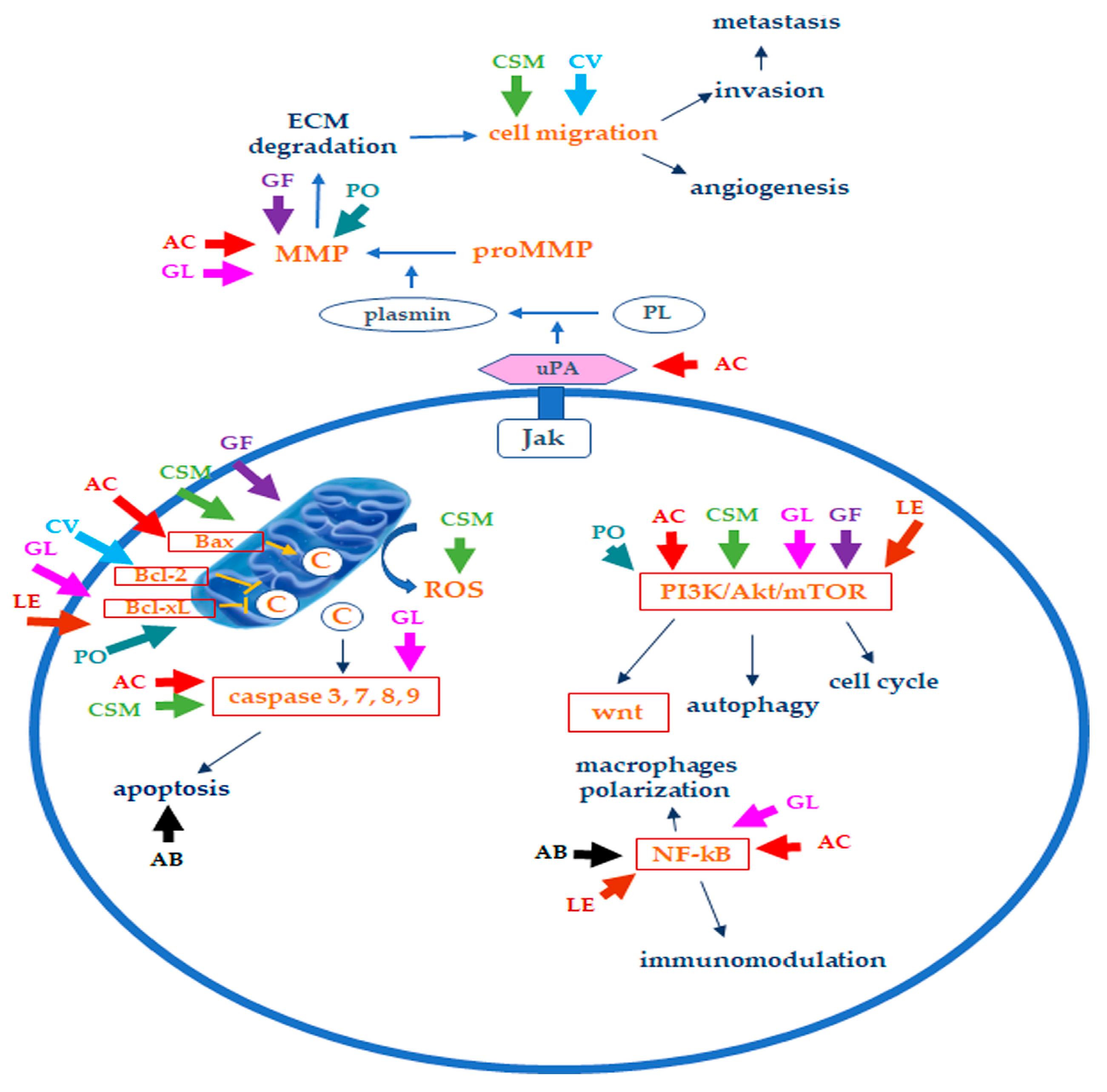
3. Edible/Medicinal Mushrooms in Breast Cancer
| Species | Main Bioactive Constituents | Mechanisms |
|---|---|---|
| Agaricus bisporum | Polysaccharides (ABP-1 and ABP-2 fractions), in particular, β-glucans (β-(1→6)-d-glucan, B16), lectins, amino acids, unsaturated fatty acids (linoleic and linolenic acids), vitamin B, vitamin C, sterols, phenolic and indole compounds, ergosterol, flavonoids, ergocalciferol, ergosterol | Inhibition of cell proliferation, suppression of tumor growth in nude mice xenografts; induction of macrophages polarization towards M1 phenotype and production of Il-6, IL-1 β, TNF-α, CoX-2; induction of nitric oxide, activation of NF-κB and cell growth inhibition, probably due to the activity on macrophages; inhibition of proteins synthesis; lectins induce cytotoxicity, apoptosis, and immune system modulation [14][21][22][23][24][25] |
| Antrodia cinnamomea | Polysaccharides, terpenoids (ergostane, lanostane), lignans, glycoproteins, benzene derivatives, ubiquinone derivatives, maleic and succinil acids derivatives, Anticin A, Antrocin C, Antcin K, antcin C, antcin B | Induction of apoptosis, suppression of mRNA expression of S-phase kinase-associated protein 2 (skp2); decrease of urokinase plasminogen activator (uPA) activity, uPA receptor (uPAR), vascular endothelial growth factor (VEGF), and MMP-9 and MMP-2; inhibition of TGF-β1-induced migration arrest epithelial to mesenchymal transition (EMT); suppression of the ERK1/2, p38, and JNK1/2 phosphorylation; inhibition of Akt/mTOR and NF-κB pathways; apoptosis induction, cell cycle arrest, antimetastatic effect, dysfunction of mitochondrial caspase-3/-9 activation, cytochrome c release, degradation of PARP, and Bcl2/Bax dysregulation; HDAC inhibition, autophagy induction (LC3-II, p62, and FOX1 increase) [12][26][27][28][29][30][31][32][33]; proliferation inhibition related to the arrest of cells at the G1 phase and induction of autophagy; stress of the endoplasmic reticulum; reduction of tumor size [32] |
| Cordyceps sinensis and Cordyceps militaris | Cordycepin (3-deoxyadenosine), ergosterol, mannitol, modifies nucleosides | Induction of apoptosis by promoting expression and translocation of Bax to mitochondria and decreasing Bcl2 levels by releasing cytochrome C, activating p53, caspase-9, caspase 3, caspase-8; inhibition of cell growth, migration, and invasion, through reduction of the EMT (TWIST1, SLUG, SNAIL1, ZEB reduction, N-cadherin downregulation, E-cadherin upregulation); inhibition of migration; antiproliferative activity through induction of apoptotic cell death; LDH release, PARP increase, ROS production, inhibition of AKT activation and PI3K/Akt; increased level of Cu/Zn superoxide dismutase in cancer cells; induction of autophagy, DNA damage, and targeting of cancer stem cells [28][34][35]; decrease in tumor weight and size; reduction of the number of metastasis; increase survival; increased expression levels of cleaved PARP, cleaved caspase-3, cleaved caspase-8, and Bax [34][36][37] |
| Coriolus versicolor | Protein-bound polysaccharides (polysaccharide peptide, PSP, and glycoprotein PSK, Krestin), terpenes, proteins, peptides, amino acids, purpurins | Suppression of cell proliferation through apoptotic cell death induction, upregulation of p53, and downregulation of Bcl-2; NK cell activation, p53, and Bcl-2 downregulation; inhibition of migration (MMP9 activity and protein levels downregulation); cytotoxicity via necroptosis activated through the TNF-α/TNFR1 pathway stimulation [38][39][40]; suppression of cancer cell proliferation, reduction of tumor weight and antimetastatic effect, simultaneously protecting bones against breast cancer-induced osteolysis; migration and invasion inhibition; immunomodulatory (increase IL-2, 6, 12 TNF-α, INF-γ, histamine, prostaglandin E) and antimigratory effects [33][41][42][43] |
| Ganoderma lucidum | Polysaccharides (α-1,3, β-1,3 and β-1,6-D-glucans, ganoderan), triterpenes, ganoderic acids, ganodermic acid, ganodermic alcohols, lucidones, lucinedic acid, ergosterol, 5,6-dehydroergosterol, ergosterol peroxide, and palmitic acid | Inhibitory effect against Akt phosphorylation on Ser473 and downregulation of Akt expression, inhibition of NF-κB, also related to estrogen receptors, cyclin D1, and subsequently cdk4 [33]; suppression of adhesion, migration, and invasion of cancer cells, down-regulation of oncogene c-myc expression and secretion of uPA and inhibition of MMP2 and MMP9 [44][45]; apoptosis induction through downregulation of cyclin F, Bcl-2, Bcl-xL and upregulation of Bax and caspase-9 levels [46][47][48]; G1 phase arrest, apoptosis induction via caspase 3/7 activation, and PARP cleavage [49]; inhibition of tumor growth and migration via inhibition of Wnt/β-catenin signaling; suppression of cancer cell growth through apoptosis induction via mitochondria-mediated pathway; effects on protein expression of E-cadherin, mammalian target of rapamycin (mTOR), human eukaryotic translation initiation factor 4G (eIF4G), and p70 ribosomal protein S6 kinase (p70S6K) and activity of extracellular regulated kinase (ERK 1/2), reduction in tumor size and weight; downregulation of immune checkpoints; effects on cancer stem cells [33][46][50][51][52][53]; reduction in incidence of mammary tumors [54] |
| Grifola Frondosa | β-glucans and α-glucan (D-fraction, X-fraction, Grifolan, MZ-fraction, and MT-α-glucan), proteins, carbohydrates, ergocalciferol, minerals | Apoptosis induction through the release of CytC from mitochondria, alterations in genes involved in cell proliferation and invasion; upregulation of E-cadherin protein levels, promotion of cell adhesion, downregulation of cell motility and MMP2 and MMP9; decrease in β-catenin levels; modulation of Bax/Bcl2 ratio, affecting the pro-survival pathways related to PI3K/Akt and ERK [28][55]; immunomodulatory effects on macrophages, NK and T cells; decrease in metastasis; inhibition of carcinogenesis, angiogenesis, and cancer invasiveness; prolonged survival [28][55] |
| Lentinula edodes | β-glucans (lentinan), phenolic compounds, ergothioneine, sterols (ergosterol), eritadenine, peptides (lenthionine) | Induction of apoptosis associated with mitochondrial membrane potential decrease and decreased cdk4 and cyclin D1 resulting in cell cycle arrest; increased p21, p53, and Bax levels; inhibition of migration, autophagy induction [33][56][57][58]; reduction in tumor growth through suppression of cell proliferation and apoptosis promotion; inhibition of multiple pathways (PI3K-Akt-mTOR, ERK, p53) [59] |
| Pleurotus ostreatus | α-glucans, β-glucans, lentanin, lipopolisaccharides, resveratrol, concavallin A, mevinolin, ergosterol | Cell growth inhibition related to cell cycle arrest at the G0/G1 phase, upregulation of the p21, p53, p27, and p19 genes and downregulation of E2f transcription factor 1, PCNA, CDK4, CDK6, and transcription factor DP-1; induction of oxidative stress and apoptotic cell death due to the upregulation of p53 and Bax, downregulation of Bcl2, and increase in caspase 3/7 activity; increased cytotoxic activity of natural killer cells; inhibition of angiogenesis and metastasis by the inhibition of MMP2 and MMP9 expression; downregulation of VEGF [28][60][61]; decrease in tumor volume and increased body weight; decrease in tumor incidence, volume, and metastasis [60][61][62] |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789.
- Porter, P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N. Engl. J. Med. 2008, 358, 213–216.
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637.
- Berretta, M.; Dal Lago, L.; Tinazzi, M.; Ronchi, A.; La Rocca, G.; Montella, L.; Di Francia, R.; Facchini, B.A.; Bignucolo, A.; Montopoli, M. Evaluation of Concomitant Use of Anticancer Drugs and Herbal Products: From Interactions to Synergic Activity. Cancers 2022, 14, 5203.
- Chan, W.-J.J.; Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V.; Harnett, J.E. Interactions between Natural Products and Cancer Treatments: Underlying Mechanisms and Clinical Importance. Cancer Chemother. Pharmacol. 2023, 91, 103–119.
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-Glucans from Grifola Frondosa and Ganoderma Lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget 2018, 9, 24837–24856.
- Habtemariam, S. The Chemistry, Pharmacology and Therapeutic Potential of the Edible Mushroom Dictyophora indusiata (Vent Ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98.
- Jiang, J.; Sliva, D. Novel Medicinal Mushroom Blend Suppresses Growth and Invasiveness of Human Breast Cancer Cells. Int. J. Oncol. 2010, 37, 1529–1536.
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; LiQun, S.; Zhong, M.T.; Huang, M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018, 17, 200–209.
- Dowaraka-Persad, B.; Neergheen, V.S. Mushroom-Derived Compounds as Metabolic Modulators in Cancer. Molecules 2023, 28, 1441.
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634.
- Popović, V.; Živković, J.; Davidović, S.; Stevanović, M.; Stojković, D. Mycotherapy of Cancer: An Update on Cytotoxic and Antitumor Activities of Mushrooms, Bioactive Principles and Molecular Mechanisms of Their Action. Curr. Top. Med. Chem. 2013, 13, 2791–2806.
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269.
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from Mushroom: Health Attributes and Food Industry Applications. Materials 2021, 14, 7640.
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Ahmad, M.Z.; Patowary, P.; Das, A. Immunomodulatory Effect of Mushrooms and Their Bioactive Compounds in Cancer: A Comprehensive Review. Biomed. Pharmacother. 2022, 149, 112901.
- Elkhateeb, W.A. What Medicinal Mushroom Can Do? Chem. Res. J. 2020, 5, 106–118.
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet and Risk of Breast, Endometrial and Ovarian Cancer: UK Women’s Cohort Study. Br. J. Nutr. 2019, 122, 564–574.
- Ba, D.M.; Ssentongo, P.; Beelman, R.B.; Muscat, J.; Gao, X.; Richie, J.P. Higher Mushroom Consumption Is Associated with Lower Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 1691–1704.
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary Mushroom Intake May Reduce the Risk of Breast Cancer: Evidence from a Meta-Analysis of Observational Studies. PLoS ONE 2014, 9, e93437.
- Chen, S.; Oh, S.-R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-Aromatase Activity of Phytochemicals in White Button Mushrooms (Agaricus Bisporus). Cancer Res. 2006, 66, 12026–12034.
- Rutckeviski, R.; Corso, C.R.; Román-Ochoa, Y.; Cipriani, T.R.; Centa, A.; Smiderle, F.R. Agaricus Bisporus β-(1 → 6)-D-Glucan Induces M1 Phenotype on Macrophages and Increases Sensitivity to Doxorubicin of Triple Negative Breast Cancer Cells. Carbohydr. Polym. 2022, 278, 118917.
- Jeong, S.C.; Koyyalamudi, S.R.; Jeong, Y.T.; Song, C.H.; Pang, G. Macrophage Immunomodulating and Antitumor Activities of Polysaccharides Isolated from Agaricus Bisporus White Button Mushrooms. J. Med. Food 2012, 15, 58–65.
- Novaes, M.R.C.G.; Valadares, F.; Reis, M.C.; Gonçalves, D.R.; Menezes, M.C. The Effects of Dietary Supplementation with Agaricales Mushrooms and Other Medicinal Fungi on Breast Cancer: Evidence-Based Medicine. Clinics 2011, 66, 2133–2139.
- Rachmawati, H.; Sundari, S.; Nabila, N.; Tandrasasmita, O.M.; Amalia, R.; Siahaan, T.J.; Tjandrawinata, R.R.; Ismaya, W.T. Orf239342 from the Mushroom Agaricus Bisporus Is a Mannose Binding Protein. Biochem. Biophys. Res. Commun. 2019, 515, 99–103.
- Chen, Y.-C.; Liu, Y.-C.; El-Shazly, M.; Wu, T.-Y.; Chang, J.-G.; Wu, Y.-C. Antrodia Cinnamomea, a Treasured Medicinal Mushroom, Induces Growth Arrest in Breast Cancer Cells, T47D Cells: New Mechanisms Emerge. Int. J. Mol. Sci. 2019, 20, 833.
- Kumar, K.J.S.; Vani, M.G.; Hsieh, H.-W.; Lin, C.-C.; Wang, S.-Y. Antcin-A Modulates Epithelial-to-Mesenchymal Transition and Inhibits Migratory and Invasive Potentials of Human Breast Cancer Cells via P53-Mediated MiR-200c Activation. Planta Med. 2019, 85, 755–765.
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom Extracts and Compounds with Suppressive Action on Breast Cancer: Evidence from Studies Using Cultured Cancer Cells, Tumor-Bearing Animals, and Clinical Trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703.
- Kumar, K.J.S.; Vani, M.G.; Chueh, P.-J.; Mau, J.-L.; Wang, S.-Y. Antrodin C Inhibits Epithelial-to-Mesenchymal Transition and Metastasis of Breast Cancer Cells via Suppression of Smad2/3 and β-Catenin Signaling Pathways. PLoS ONE 2015, 10, e117111.
- Yang, H.-L.; Kuo, Y.-H.; Tsai, C.-T.; Huang, Y.-T.; Chen, S.-C.; Chang, H.-W.; Lin, E.; Lin, W.-H.; Hseu, Y.-C. Anti-Metastatic Activities of Antrodia Camphorata against Human Breast Cancer Cells Mediated through Suppression of the MAPK Signaling Pathway. Food Chem. Toxicol. 2011, 49, 290–298.
- Yang, H.-L.; Chen, C.-S.; Chang, W.-H.; Lu, F.-J.; Lai, Y.-C.; Chen, C.-C.; Hseu, T.-H.; Kuo, C.-T.; Hseu, Y.-C. Growth Inhibition and Induction of Apoptosis in MCF-7 Breast Cancer Cells by Antrodia Camphorata. Cancer Lett. 2006, 231, 215–227.
- Lin, Y.-S.; Lin, Y.-Y.; Yang, Y.-H.; Lin, C.-L.; Kuan, F.-C.; Lu, C.-N.; Chang, G.-H.; Tsai, M.-S.; Hsu, C.-M.; Yeh, R.-A.; et al. Antrodia Cinnamomea Extract Inhibits the Proliferation of Tamoxifen-Resistant Breast Cancer Cells through Apoptosis and Skp2/MicroRNAs Pathway. BMC Complement. Altern. Med. 2018, 18, 152.
- Nowakowski, P.; Markiewicz-Żukowska, R.; Bielecka, J.; Mielcarek, K.; Grabia, M.; Socha, K. Treasures from the Forest: Evaluation of Mushroom Extracts as Anti-Cancer Agents. Biomed. Pharmacother. 2021, 143, 112106.
- Wei, C.; Khan, M.A.; Du, J.; Cheng, J.; Tania, M.; Leung, E.L.-H.; Fu, J. Cordycepin Inhibits Triple-Negative Breast Cancer Cell Migration and Invasion by Regulating EMT-TFs SLUG, TWIST1, SNAIL1, and ZEB1. Front. Oncol. 2022, 12, 898583.
- Lee, D.; Lee, W.-Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.-E.; Lee, S.; Kang, K.S. The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules 2019, 9, 407.
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study Using 4t1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479.
- Song, J.; Wang, Y.; Teng, M.; Zhang, S.; Yin, M.; Lu, J.; Liu, Y.; Lee, R.J.; Wang, D.; Teng, L. Cordyceps Militaris Induces Tumor Cell Death via the Caspase-Dependent Mitochondrial Pathway in HepG2 and MCF-7 Cells. Mol. Med. Rep. 2016, 13, 5132–5140.
- Habtemariam, S. Trametes Versicolor (Synn. Coriolus Versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135.
- Ho, C.-Y.; Kim, C.-F.; Leung, K.-N.; Fung, K.-P.; Tse, T.-F.; Chan, H.; Lau, C.B.-S. Differential Anti-Tumor Activity of Coriolus Versicolor (Yunzhi) Extract through P53- and/or Bcl-2-Dependent Apoptotic Pathway in Human Breast Cancer Cells. Cancer Biol. Ther. 2005, 4, 638–644.
- Aoyagi, H.; Iino, Y.; Takeo, T.; Horii, Y.; Morishita, Y.; Horiuchi, R. Effects of OK-432 (Picibanil) on the Estrogen Receptors of MCF-7 Cells and Potentiation of Antiproliferative Effects of Tamoxifen in Combination with OK-432. Oncology 1997, 54, 414–423.
- Luo, K.-W.; Yue, G.G.-L.; Ko, C.-H.; Lee, J.K.-M.; Gao, S.; Li, L.-F.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. In Vivo and in Vitro Anti-Tumor and Anti-Metastasis Effects of Coriolus Versicolor Aqueous Extract on Mouse Mammary 4T1 Carcinoma. Phytomedicine 2014, 21, 1078–1087.
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus Versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063.
- Pawlikowska, M.; Jadrzejewski, T.; Broayna, A.A.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Induce RIPK1/RIPK3/MLKL-Mediated Necroptosis in ER-Positive Breast Cancer and Amelanotic Melanoma Cells. Cell. Physiol. Biochem. 2020, 54, 591–604.
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma Lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, 210.
- Loganathan, J.; Jiang, J.; Smith, A.; Jedinak, A.; Thyagarajan-Sahu, A.; Sandusky, G.E.; Nakshatri, H.; Sliva, D. The Mushroom Ganoderma Lucidum Suppresses Breast-to-Lung Cancer Metastasis through the Inhibition of pro-Invasive Genes. Int. J. Oncol. 2014, 45, 2009–2015.
- Suarez-Arroyo, I.J.; Rosario-Acevedo, R.; Aguilar-Perez, A.; Clemente, P.L.; Cubano, L.A.; Serrano, J.; Schneider, R.J.; Martínez-Montemayor, M.M. Anti-Tumor Effects of Ganoderma Lucidum (Reishi) in Inflammatory Breast Cancer in In Vivo and In Vitro Models. PLoS ONE 2013, 8, e57431.
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic Acids Suppress Growth and Invasive Behavior of Breast Cancer Cells by Modulating AP-1 and NF-ΚB Signaling. Int. J. Mol. Med. 2008, 21, 577–584.
- Lu, Q.-Y.; Sartippour, M.R.; Brooks, M.N.; Zhang, Q.; Hardy, M.; Go, V.L.; Li, F.P.; Heber, D. Ganoderma Lucidum Spore Extract Inhibits Endothelial and Breast Cancer Cells in Vitro. Oncol. Rep. 2004, 12, 659–662.
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma Lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-Cancer Activities in Vitro. Front. Pharmacol. 2019, 10, 115.
- Suárez-Arroyo, I.J.; Rios-Fuller, T.J.; Feliz-Mosquea, Y.R.; Lacourt-Ventura, M.; Leal-Alviarez, D.J.; Maldonado-Martinez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Ganoderma Lucidum Combined with the EGFR Tyrosine Kinase Inhibitor, Erlotinib Synergize to Reduce Inflammatory Breast Cancer Progression. J. Cancer 2016, 7, 500–511.
- Martínez-Montemayor, M.M.; Acevedo, R.R.; Otero-Franqui, E.; Cubano, L.A.; Dharmawardhane, S.F. Ganoderma Lucidum (Reishi) Inhibits Cancer Cell Growth and Expression of Key Molecules in Inflammatory Breast Cancer. Nutr. Cancer 2011, 63, 1085–1094.
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor Activity of Extract From the Sporoderm-Breaking Spore of Ganoderma Lucidum: Restoration on Exhausted Cytotoxic T Cell With Gut Microbiota Remodeling. Front. Immunol. 2018, 9, 1765.
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma Lucidum Extract (GLE) Impairs Breast Cancer Stem Cells by Targeting the STAT3 Pathway. Oncotarget 2018, 9, 35907–35921.
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.A.; Janardhanan, K.K. Ganoderma Lucidum Total Triterpenes Induce Apoptosis in MCF-7 Cells and Attenuate DMBA Induced Mammary and Skin Carcinomas in Experimental Animals. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2017, 813, 45–51.
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and Antimetastatic Activity of Maitake D-Fraction in Triple-Negative Breast Cancer Cells. Oncotarget 2018, 9, 23396–23412.
- Din, S.R.U.; Zhong, M.; Nisar, M.A.; Saleem, M.Z.; Hussain, A.; Khinsar, K.H.; Alam, S.; Ayub, G.; Kanwal, S.; Li, X.; et al. Latcripin-7A, Derivative of Lentinula Edodes C91–3, Reduces Migration and Induces Apoptosis, Autophagy, and Cell Cycle Arrest at G1 Phase in Breast Cancer Cells. Appl. Microbiol. Biotechnol. 2020, 104, 10165–10179.
- Israilides, C.; Kletsas, D.; Arapoglou, D.; Philippoussis, A.; Pratsinis, H.; Ebringerová, A.; Hříbalová, V.; Harding, S.E. In Vitro Cytostatic and Immunomodulatory Properties of the Medicinal Mushroom Lentinula edodes. Phytomedicine 2008, 15, 512–519.
- Fang, N.; Li, Q.; Yu, S.; Zhang, J.; He, L.; Ronis, M.J.J.; Badger, T.M. Inhibition of Growth and Induction of Apoptosis in Human Cancer Cell Lines by an Ethyl Acetate Fraction from Shiitake Mushrooms. J. Altern. Complement. Med. 2006, 12, 125–132.
- Xu, H.; Zou, S.; Xu, X. The β-Glucan from Lentinus Edodes Suppresses Cell Proliferation and Promotes Apoptosis in Estrogen Receptor Positive Breast Cancers. Oncotarget 2017, 8, 86693–86709.
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising Anticancer Activity of Polysaccharides and Other Macromolecules Derived from Oyster Mushroom (Pleurotus Sp.): An Updated Review. Int. J. Biol. Macromol. 2021, 182, 1628–1637.
- Krishnamoorthy, D.; Sankaran, M. Modulatory Effect of Pleurotus Ostreatus on Oxidant/Antioxidant Status in 7, 12-Dimethylbenz (a) Anthracene Induced Mammary Carcinoma in Experimental Rats—A Dose-Response Study. J. Cancer Res. Ther. 2016, 12, 386–394.
- Haque, M.D.; Islam, M.D. Pleurotus Highking Mushroom Induces Apoptosis by Altering the Balance of Proapoptotic and Antiapoptotic Genes in Breast Cancer Cells and Inhibits Tumor Sphere Formation. Medicina 2019, 55, 716.




