Edible/medicinal mushrooms have been traditionally used in Asian countries either in the cuisine or as dietary supplements and nutraceuticals. Among the different pharmacological activities reported (antibacterial, anti-inflammatory, antioxidative, antiviral, immunomodulating, antidiabetic, etc.), edible/medicinal mushrooms have been shown to exert in vitro and in vivo anticancer effects on several kinds of tumors, including breast cancer.
- breast cancer
- edible/medicinal mushrooms
1. Introduction
Breast cancer (BC) is among the major causes of cancer-related deaths worldwide, with 2.3 million new cases per year, according to the GLOBOCAN 2020 data [1]. Projections indicate that by 2030, the number of new cases diagnosed worldwide will reach 2.7 million, while the number of deaths will reach 0.9 million [2] (Global Cancer Observatory: Cancer Tomorrow, accessed on 29 April 2023. Available online: https://gco.iarc.fr/tomorrow). In addition, the incidence of breast cancer is expected to further increase, in particular in low- and medium-income countries, due to the effects of a westernized lifestyle, characterized by delayed pregnancies, reduced breastfeeding, low age at menarche, lack of physical activity, and poor diet [3].
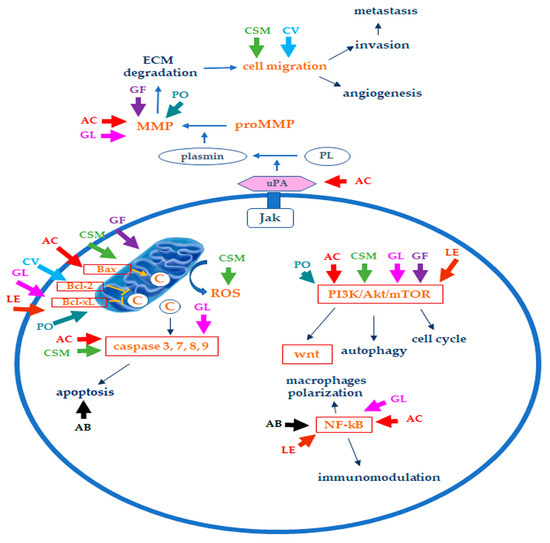
2. Bioactive Compounds in Medicinal Mushrooms and Their Mechanisms of Action
3. Edible/Medicinal Mushrooms in Breast Cancer
| Species | Main Bioactive Constituents | Mechanisms |
|---|---|---|
| Agaricus bisporum | Polysaccharides (ABP-1 and ABP-2 fractions), in particular, β-glucans (β-(1→6)-d-glucan, B16), lectins, amino acids, unsaturated fatty acids (linoleic and linolenic acids), vitamin B, vitamin C, sterols, phenolic and indole compounds, ergosterol, flavonoids, ergocalciferol, ergosterol | Inhibition of cell proliferation, suppression of tumor growth in nude mice xenografts; induction of macrophages polarization towards M1 phenotype and production of Il-6, IL-1 β, TNF-α, CoX-2; induction of nitric oxide, activation of NF-κB and cell growth inhibition, probably due to the activity on macrophages; inhibition of proteins synthesis; lectins induce cytotoxicity, apoptosis, and immune system modulation [33,47,48,49,50,51] |
| Antrodia cinnamomea | Polysaccharides, terpenoids (ergostane, lanostane), lignans, glycoproteins, benzene derivatives, ubiquinone derivatives, maleic and succinil acids derivatives, Anticin A, Antrocin C, Antcin K, antcin C, antcin B | Induction of apoptosis, suppression of mRNA expression of S-phase kinase-associated protein 2 (skp2); decrease of urokinase plasminogen activator (uPA) activity, uPA receptor (uPAR), vascular endothelial growth factor (VEGF), and MMP-9 and MMP-2; inhibition of TGF-β1-induced migration arrest epithelial to mesenchymal transition (EMT); suppression of the ERK1/2, p38, and JNK1/2 phosphorylation; inhibition of Akt/mTOR and NF-κB pathways; apoptosis induction, cell cycle arrest, antimetastatic effect, dysfunction of mitochondrial caspase-3/-9 activation, cytochrome c release, degradation of PARP, and Bcl2/Bax dysregulation; HDAC inhibition, autophagy induction (LC3-II, p62, and FOX1 increase) [31,53,57,58,59,60,61,62,126]; proliferation inhibition related to the arrest of cells at the G1 phase and induction of autophagy; stress of the endoplasmic reticulum; reduction of tumor size [62] |
| Cordyceps sinensis and Cordyceps militaris | Cordycepin (3-deoxyadenosine), ergosterol, mannitol, modifies nucleosides | Induction of apoptosis by promoting expression and translocation of Bax to mitochondria and decreasing Bcl2 levels by releasing cytochrome C, activating p53, caspase-9, caspase 3, caspase-8; inhibition of cell growth, migration, and invasion, through reduction of the EMT (TWIST1, SLUG, SNAIL1, ZEB reduction, N-cadherin downregulation, E-cadherin upregulation); inhibition of migration; antiproliferative activity through induction of apoptotic cell death; LDH release, PARP increase, ROS production, inhibition of AKT activation and PI3K/Akt; increased level of Cu/Zn superoxide dismutase in cancer cells; induction of autophagy, DNA damage, and targeting of cancer stem cells [58,63,65]; decrease in tumor weight and size; reduction of the number of metastasis; increase survival; increased expression levels of cleaved PARP, cleaved caspase-3, cleaved caspase-8, and Bax [63,66,67] |
| Coriolus versicolor | Protein-bound polysaccharides (polysaccharide peptide, PSP, and glycoprotein PSK, Krestin), terpenes, proteins, peptides, amino acids, purpurins | Suppression of cell proliferation through apoptotic cell death induction, upregulation of p53, and downregulation of Bcl-2; NK cell activation, p53, and Bcl-2 downregulation; inhibition of migration (MMP9 activity and protein levels downregulation); cytotoxicity via necroptosis activated through the TNF-α/TNFR1 pathway stimulation [77,78,79]; suppression of cancer cell proliferation, reduction of tumor weight and antimetastatic effect, simultaneously protecting bones against breast cancer-induced osteolysis; migration and invasion inhibition; immunomodulatory (increase IL-2, 6, 12 TNF-α, INF-γ, histamine, prostaglandin E) and antimigratory effects [80,81,82,126] |
| Ganoderma lucidum | Polysaccharides (α-1,3, β-1,3 and β-1,6-D-glucans, ganoderan), triterpenes, ganoderic acids, ganodermic acid, ganodermic alcohols, lucidones, lucinedic acid, ergosterol, 5,6-dehydroergosterol, ergosterol peroxide, and palmitic acid | Inhibitory effect against Akt phosphorylation on Ser473 and downregulation of Akt expression, inhibition of NF-κB, also related to estrogen receptors, cyclin D1, and subsequently cdk4 [126]; suppression of adhesion, migration, and invasion of cancer cells, down-regulation of oncogene c-myc expression and secretion of uPA and inhibition of MMP2 and MMP9 [92,98]; apoptosis induction through downregulation of cyclin F, Bcl-2, Bcl-xL and upregulation of Bax and caspase-9 levels [91,94,95]; G1 phase arrest, apoptosis induction via caspase 3/7 activation, and PARP cleavage [89]; inhibition of tumor growth and migration via inhibition of Wnt/β-catenin signaling; suppression of cancer cell growth through apoptosis induction via mitochondria-mediated pathway; effects on protein expression of E-cadherin, mammalian target of rapamycin (mTOR), human eukaryotic translation initiation factor 4G (eIF4G), and p70 ribosomal protein S6 kinase (p70S6K) and activity of extracellular regulated kinase (ERK 1/2), reduction in tumor size and weight; downregulation of immune checkpoints; effects on cancer stem cells [91,99,100,106,126,127]; reduction in incidence of mammary tumors [96] |
| Grifola Frondosa | β-glucans and α-glucan (D-fraction, X-fraction, Grifolan, MZ-fraction, and MT-α-glucan), proteins, carbohydrates, ergocalciferol, minerals | Apoptosis induction through the release of CytC from mitochondria, alterations in genes involved in cell proliferation and invasion; upregulation of E-cadherin protein levels, promotion of cell adhesion, downregulation of cell motility and MMP2 and MMP9; decrease in β-catenin levels; modulation of Bax/Bcl2 ratio, affecting the pro-survival pathways related to PI3K/Akt and ERK [58,109]; immunomodulatory effects on macrophages, NK and T cells; decrease in metastasis; inhibition of carcinogenesis, angiogenesis, and cancer invasiveness; prolonged survival [58,109] |
| Lentinula edodes | β-glucans (lentinan), phenolic compounds, ergothioneine, sterols (ergosterol), eritadenine, peptides (lenthionine) | Induction of apoptosis associated with mitochondrial membrane potential decrease and decreased cdk4 and cyclin D1 resulting in cell cycle arrest; increased p21, p53, and Bax levels; inhibition of migration, autophagy induction [115,116,117,126]; reduction in tumor growth through suppression of cell proliferation and apoptosis promotion; inhibition of multiple pathways (PI3K-Akt-mTOR, ERK, p53) [118] |
| Pleurotus ostreatus | α-glucans, β-glucans, lentanin, lipopolisaccharides, resveratrol, concavallin A, mevinolin, ergosterol | Cell growth inhibition related to cell cycle arrest at the G0/G1 phase, upregulation of the p21, p53, p27, and p19 genes and downregulation of E2f transcription factor 1, PCNA, CDK4, CDK6, and transcription factor DP-1; induction of oxidative stress and apoptotic cell death due to the upregulation of p53 and Bax, downregulation of Bcl2, and increase in caspase 3/7 activity; increased cytotoxic activity of natural killer cells; inhibition of angiogenesis and metastasis by the inhibition of MMP2 and MMP9 expression; downregulation of VEGF [58,121,124]; decrease in tumor volume and increased body weight; decrease in tumor incidence, volume, and metastasis [121,124,125] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms241210120
