
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga Mediano | -- | 3370 | 2023-06-21 10:18:19 | | | |
| 2 | Camila Xu | Meta information modification | 3370 | 2023-06-21 10:31:20 | | |
Video Upload Options
Obstructive sleep apnea (OSA) in children is a prevalent, but still, today, underdiagnosed illness, which consists of repetitive episodes of upper airway obstruction during sleep with important repercussions for sleep quality. OSA has relevant consequences in the pediatric population, mainly in the metabolic, cardiovascular (CV), and neurological spheres. However, contrary to adults, advances in diagnostic and therapeutic management have been scarce in the last few years despite the increasing scientific evidence of the deleterious consequences of pediatric OSA. The problem of underdiagnosis and the lack of response to treatment in some groups make an update to the management of OSA in children necessary. Probably, the heterogeneity of OSA is not well represented by the classical clinical presentation and severity parameters (apnea/hypopnea index (AHI)), and new strategies are required. A specific and consensus definition should be established. Additionally, the role of simplified methods in the diagnosis algorithm should be considered. Finally, the search for new biomarkers for risk stratification is needed in this population. In conclusion, new paradigms based on personalized medicine should be implemented in this population.
1. Definition and Prevalence of Obstructive Sleep Apnea in Children
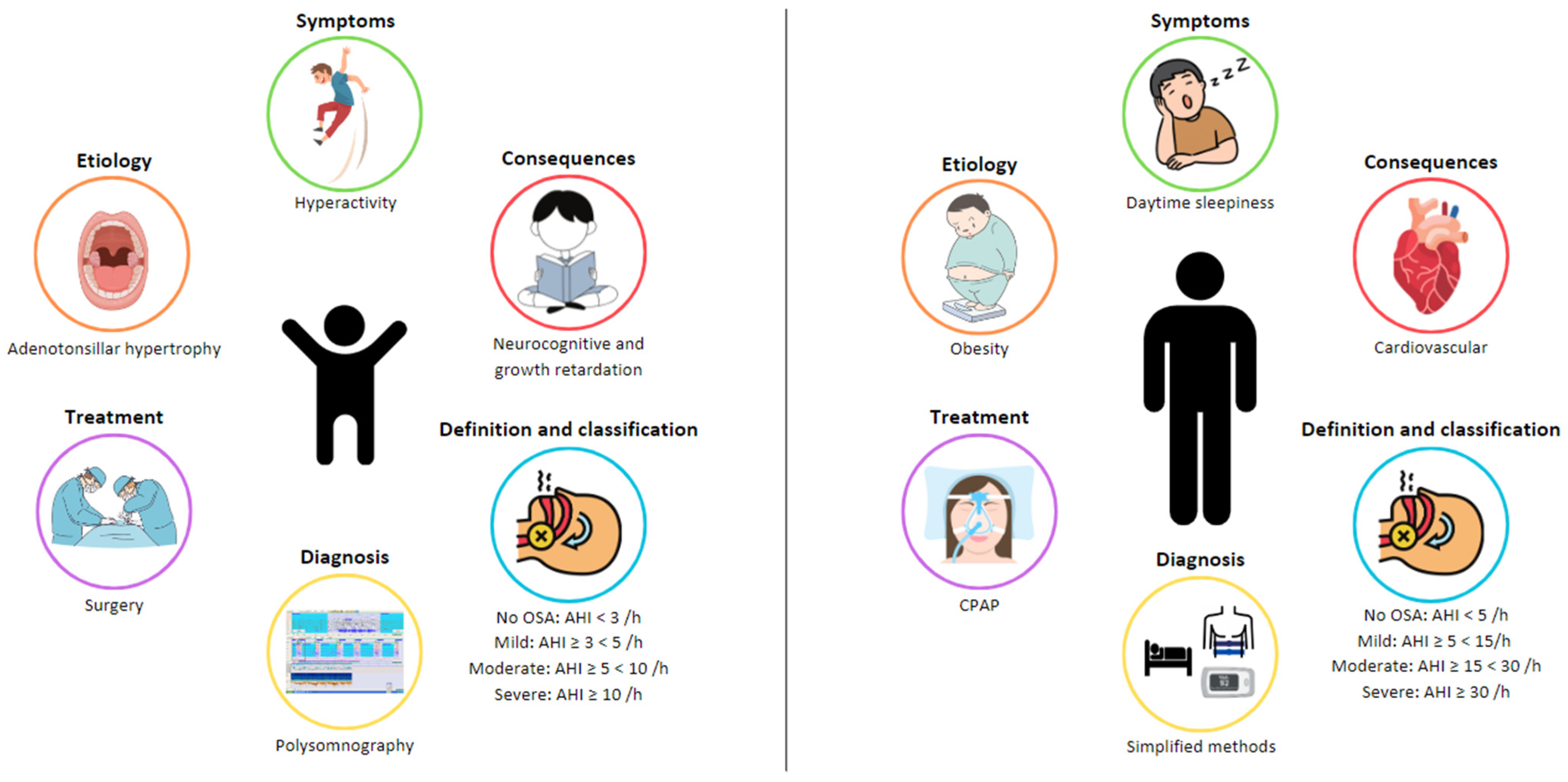
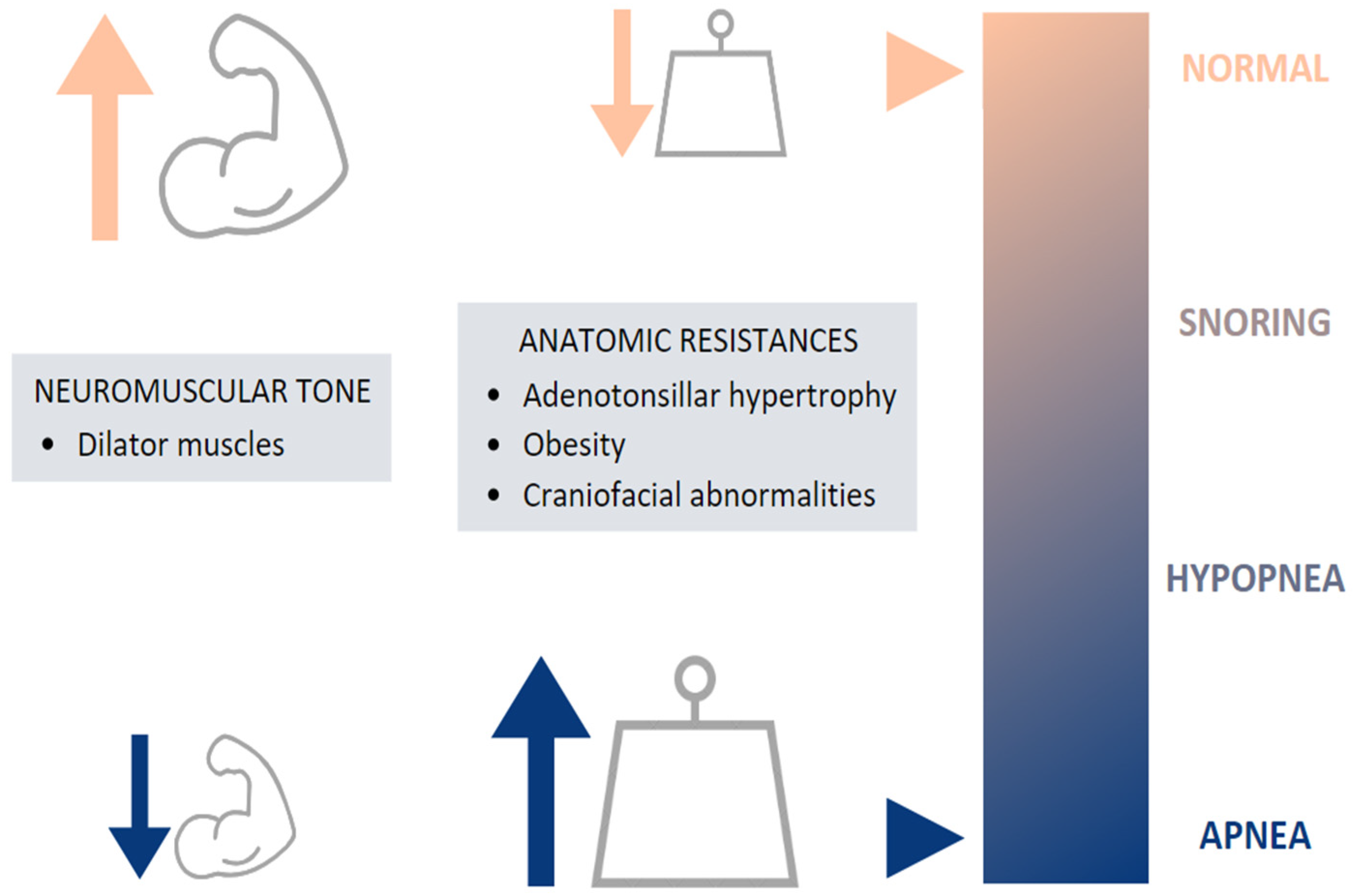
2. Etiology of OSA in Children
3. Symptoms of OSA in Children
| Nocturnal Symptoms | Daytime Symptoms |
|---|---|
| Snoring | Behavioral disorders |
| Witnessed apneas | Neurocognitive disorders |
| Gasping | Mood instability |
| Oral breathing | Excessive daytime sleepiness |
| Paradoxical thoracic movements | |
| Nightmares | |
| Restless sleep | |
| Nocturnal enuresis |
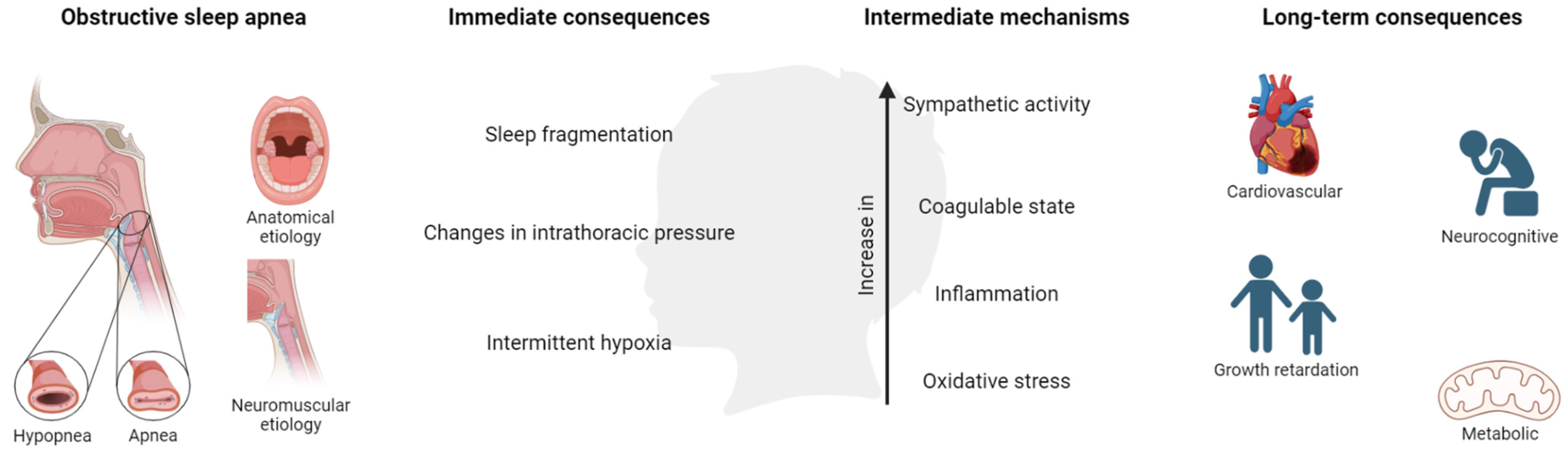
4. Consequences
| Author (Year) | Number of Participants | Age (Years) | OSA Severity Criteria | Outcomes | Results |
|---|---|---|---|---|---|
| Behavioral and neurocognitive sphere | |||||
| Menzies et al., 2022 [38] Metanalysis of 63 studies |
17,834 | From 2 to 18 years | Due to the lack of a consensus severity criterion, the subgroup given by the author was used (e.g., mild OSA) | Intelligence, attention, memory, visual spatial skills, and language | Children with SDB had significant impairments in all cognitive domains, intelligence being the most-affected quality. These neurocognitive deficits were found in primary snorers among OSA children. |
| Growth retardation and metabolism | |||||
| Lagravère et al., 2019 [39] Systematic review of 12 studies |
Growth mediators (IGF-I and IGFBP-3) | Children with OSA present lower levels of growth mediators, indicating growth retardation, significantly higher cardiovascular disease risk, and decreased cognitive functions compared to healthy controls. Tonsillectomy may improve all these functions with a great impact on general health. |
|||
| Cardiovascular sphere | |||||
| Ai et al., 2022 [40] Metanalysis of 14 studies |
3081 | 3 to 17 years | Mild OSA is defined as an AHI between 1 and 5 events per hour Moderate to severe is defined as an AHI ≥ 5 /h. |
BP parameters: awake and nighttime SBP and DBP | The mean SBP was higher in children with mild or moderate-to-severe OSA compared to healthy controls, these effects being more pronounced during the night. The results suggest that moderate-to-severe OSA in children is associated with a higher risk of adverse SBP outcomes. |
5. Diagnosis
| Guide | Diagnosis and Management of OSA in Children |
|---|---|
| Spanish Society of Pneumology and Thoracic Surgery (SEPAR) | This guide divides the OSA diagnostic methodology between primary care and hospital care in order to increase the diagnostic efficiency. In primary care, the evaluation of the child with suspected OSA (presence of snoring and symptoms or suggestive clinical findings) should include the medical history and complete clinical examination.
|
| European Respiratory Society | The diagnosis and management for SDB is described as a stepwise approach in 7 steps.
|
| American Academy of Pediatrics | This practice guideline focuses on uncomplicated childhood OSA, associated with adenotonsillar hypertrophy and/or obesity in an otherwise child who is being treated in the primary care setting. It comprises 8 key action statements.
|
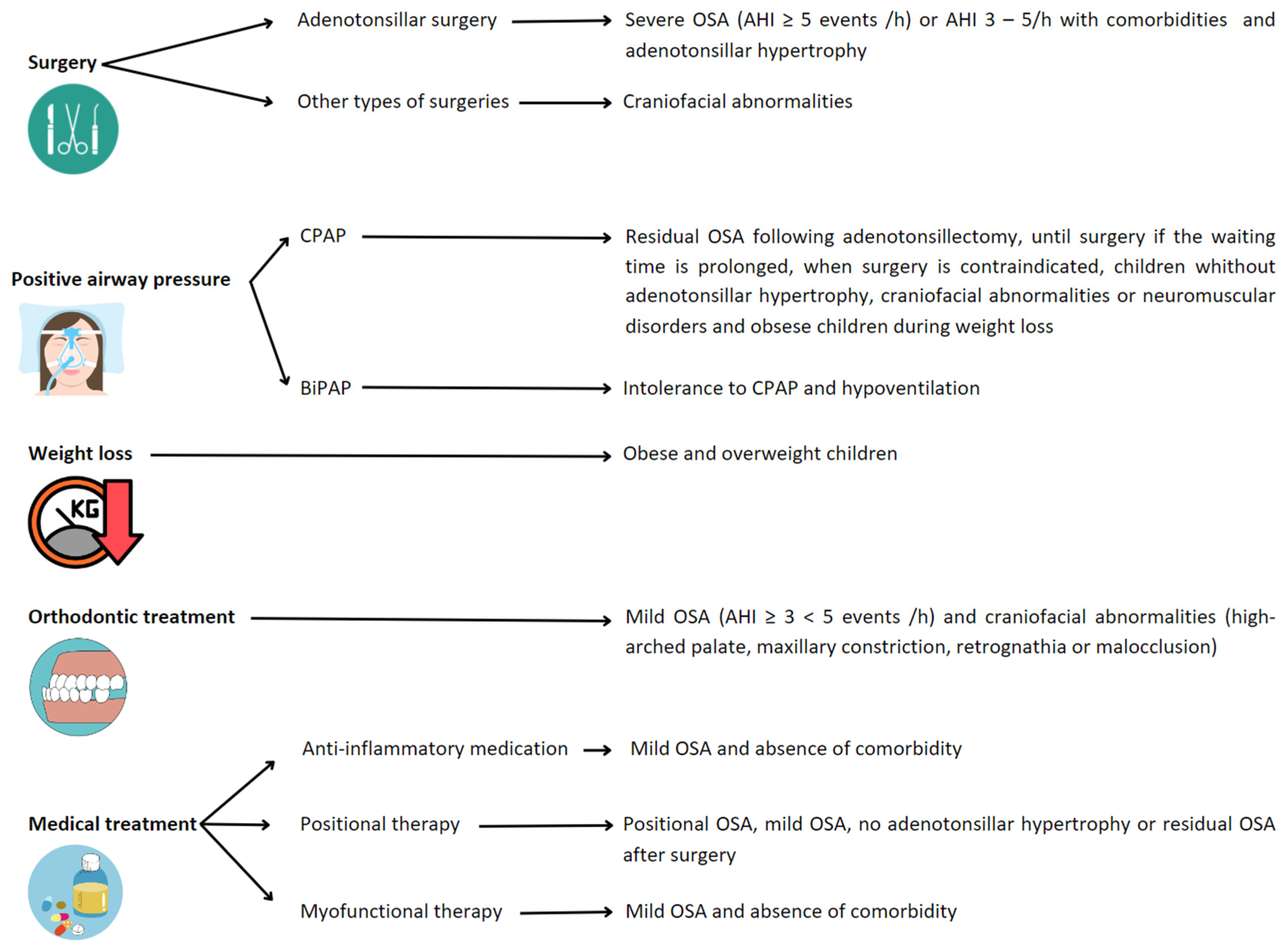
6. Treatment
References
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive Sleep Disordered Breathing in 2- to 18-Year-Old Children: Diagnosis and Management. Eur. Respir. J. 2016, 47, 69–94.
- Gozal, D.; O’Brien, L.M. Snoring and Obstructive Sleep Apnoea in Children: Why Should We Treat? Paediatr. Respir. Rev. 2004, 5, S371–S376.
- Marcus, C.L.; Brooks, L.J.; Ward, S.D.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Lehmann, C.; Schechter, M.S.; Sheldon, S.; et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics 2012, 130, e714–e755.
- Iber, C.; Ancoli-Israel, S.; Chesson, A.L.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; Volume 1.
- Luz Alonso-Álvarez, M.; Canet, T.; Cubell-Alarco, M.; Estivill, E.; Fernández-Julián, E.; Gozal, D.; Jurado-Luque, M.J.; Lluch-Roselló, M.A.; Martínez-Pérez, F.; Merino-Andreu, M.; et al. Documento de consenso del síndrome de apneas-hipopneas durante el sueño en niños (versión completa). Arch. Bronconeumol. 2011, 47, 2–18.
- Witmans, M.; Tablizo, M.A. Current Concepts in Pediatric Obstructive Sleep Apnea. Children 2023, 10, 480.
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270.
- Dehlink, E.; Tan, H.-L. Update on Paediatric Obstructive Sleep Apnoea. J. Thorac. Dis. 2016, 8, 224–235.
- Lo Bue, A.; Salvaggio, A.; Insalaco, G. Obstructive Sleep Apnea in Developmental Age. A Narrative Review. Eur. J. Pediatr. 2020, 179, 357–365.
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; de Vincentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk Factors for Obstructive Sleep Apnea Syndrome in Children: State of the Art. Int. J. Environ. Res. Public Health 2019, 16, 3235.
- Al-Shamrani, A.; Alharbi, A.S. Diagnosis and Management of Childhood Sleep-Disordered Breathing: Clinical Approach. Saudi Med. J. 2020, 41, 916–929.
- Lin, S.; Su, Y.; Wu, Y.; Chang, J.Z.; Tu, Y. Management of Paediatric Obstructive Sleep Apnoea: A Systematic Review and Network Meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 156–170.
- Lam, Y.; Chan, E.Y.T.; Ng, D.K.; Chan, C.; Cheung, J.M.Y.; Leung, S.; Chow, P.; Kwok, K. The Correlation Among Obesity, Apnea-Hypopnea Index, and Tonsil Size in Children. Chest 2006, 130, 1751–1756.
- Wang, J.; Zhao, Y.; Yang, W.; Shen, T.; Xue, P.; Yan, X.; Chen, D.; Qiao, Y.; Chen, M.; Ren, R.; et al. Correlations between Obstructive Sleep Apnea and Adenotonsillar Hypertrophy in Children of Different Weight Status. Sci. Rep. 2019, 9, 11455.
- Li, J.; Yang, Q.; Xu, Y.; Han, F.; Zhao, J. Research progress on correlation between childhood obesity and obstructive sleep apnea. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2023, 37, 318–322.
- Narang, I.; Mathew, J.L. Childhood Obesity and Obstructive Sleep Apnea. J. Nutr. Metab. 2012, 2012, 134202.
- Lee, J.H.; Cho, J. Sleep and Obesity. Sleep Med. Clin. 2022, 17, 111–116.
- Goodwin, J.L.; Vasquez, M.M.; Silva, G.E.; Quan, S.F. Incidence and Remission of Sleep-Disordered Breathing and Related Symptoms in 6- to 17-Year Old Children—The Tucson Children’s Assessment of Sleep Apnea Study. J. Pediatr. 2010, 157, 57–61.
- Savini, S.; Ciorba, A.; Bianchini, C.; Stomeo, F.; Corazzi, V.; Vicini, C.; Pelucchi, S. Assessment of Obstructive Sleep Apnoea (OSA) in Children: An Update. Acta Otorhinolaryngol. Ital. 2019, 39, 289–297.
- Zaffanello, M.; Piacentini, G.; Lippi, G.; Fanos, V.; Gasperi, E.; Nosetti, L. Obstructive Sleep-Disordered Breathing, Enuresis and Combined Disorders in Children: Chance or Related Association? Swiss Med. Wkly. 2017, 147, w14400.
- Penzel, T.; Hornero, R. (Eds.) Advances in the Diagnosis and Treatment of Sleep Apnea: Filling the Gap between Physicians and Engineers; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; Volume 1384, ISBN 978-3-031-06412-8.
- Beebe, D.W.; Rausch, J.; Byars, K.C.; Lanphear, B.; Yolton, K. Persistent Snoring in Preschool Children: Predictors and Behavioral and Developmental Correlates. Pediatrics 2012, 130, 382–389.
- Bucks, R.S.; Olaithe, M.; Eastwood, P. Neurocognitive Function in Obstructive Sleep Apnoea: A Meta-Review: Cognitive Function in OSA: A Meta-Review. Respirology 2013, 18, 61–70.
- Urschitz, M.S.; Guenther, A.; Eggebrecht, E.; Wolff, J.; Urschitz-Duprat, P.M.; Schlaud, M.; Poets, C.F. Snoring, Intermittent Hypoxia and Academic Performance in Primary School Children. Am. J. Respir. Crit. Care Med. 2003, 168, 464–468.
- Galland, B.; Spruyt, K.; Dawes, P.; McDowall, P.S.; Elder, D.; Schaughency, E. Sleep Disordered Breathing and Academic Performance: A Meta-Analysis. Pediatrics 2015, 136, e934–e946.
- Brockmann, P.E.; Gozal, D. Neurocognitive Consequences in Children with Sleep Disordered Breathing: Who Is at Risk? Children 2022, 9, 1278.
- Bonuck, K.A.; Freeman, K.; Henderson, J. Growth and Growth Biomarker Changes after Adenotonsillectomy: Systematic Review and Meta-Analysis. Arch. Dis. Child. 2008, 94, 83–91.
- Esteller, E.; Villatoro, J.C.; Agüero, A.; Lopez, R.; Matiñó, E.; Argemi, J.; Girabent-Farrés, M. Obstructive Sleep Apnea Syndrome and Growth Failure. Int. J. Pediatr. Otorhinolaryngol. 2018, 108, 214–218.
- Gümüssoy, M.; Atmaca, S.; Bilgici, B.; Ünal, R. Changes in IGF-I, IGFBP-3 and Ghrelin Levels after Adenotonsillectomy in Children with Sleep Disordered Breathing. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1653–1656.
- Bhattacharjee, R.; Kheirandish-Gozal, L.; Pillar, G.; Gozal, D. Cardiovascular Complications of Obstructive Sleep Apnea Syndrome: Evidence from Children. Prog. Cardiovasc. Dis. 2009, 51, 416–433.
- Kang, K.-T.; Weng, W.-C.; Lee, P.-L.; Hsu, W.-C. C-Reactive Protein in Children with Obstructive Sleep Apnea and Effects of Adenotonsillectomy. Auris Nasus Larynx 2022, 49, 92–99.
- Smith, D.F.; Amin, R.S. OSA and Cardiovascular Risk in Pediatrics. Chest 2019, 156, 402–413.
- Amin, R.S.; Kimball, T.R.; Kalra, M.; Jeffries, J.L.; Carroll, J.L.; Bean, J.A.; Witt, S.A.; Glascock, B.J.; Daniels, S.R. Left Ventricular Function in Children with Sleep-Disordered Breathing. Am. J. Cardiol. 2005, 95, 801–804.
- Nemati, S.; Aghajankhah, M.; Banan, R.; Haddadi, S.; Mehri, M.; Aghsaghloo, V.; Leili, E.K. The Effects of Adeno/Tonsillectomy on Cardiopulmonary Function Based on Echocardiography Indices in Children with Primary Snoring and Mild Obstructive Sleep Apnea. Am. J. Otolaryngol. 2022, 43, 103317.
- Sameema, V.V.; Soni, K.; Deora, S.; Sharma, J.B.; Choudhury, B.; Kaushal, D.; Chhabra, S.; Goyal, A. Assessment of Preoperative and Postoperative Cardiac Function in Children with Adenotonsillar Hypertrophy: A Prospective Cohort Study. Eur. Arch. Otorhinolaryngol. 2022, 279, 3013–3019.
- Giuca, M.R.; Carli, E.; Lardani, L.; Pasini, M.; Miceli, M.; Fambrini, E. Pediatric Obstructive Sleep Apnea Syndrome: Emerging Evidence and Treatment Approach. Sci. World J. 2021, 2021, 5591251.
- Redline, S.; Storfer-Isser, A.; Rosen, C.L.; Johnson, N.L.; Kirchner, H.L.; Emancipator, J.; Kibler, A.M. Association between Metabolic Syndrome and Sleep-Disordered Breathing in Adolescents. Am. J. Respir. Crit. Care Med. 2007, 176, 401–408.
- Menzies, B.; Teng, A.; Burns, M.; Lah, S. Neurocognitive Outcomes of Children with Sleep Disordered Breathing: A Systematic Review with Meta-Analysis. Sleep Med. Rev. 2022, 63, 101629.
- Lagravère, M.O.; Zecca, P.A.; Caprioglio, A.; Fastuca, R. Metabolic Effects of Treatment in Patients with Obstructive Sleep Apnea: A Systematic Review. Minerva Pediatr. 2019, 71, 380–389.
- Ai, S.; Li, Z.; Wang, S.; Chen, S.; Chan, J.W.; Au, C.T.; Bao, Y.; Li, A.M.; Zhang, J.; Chan, K.C.-C.; et al. Blood Pressure and Childhood Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2022, 65, 101663.
- Thomas, S.; Patel, S.; Gummalla, P.; Tablizo, M.A.; Kier, C. You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children 2022, 9, 261.
- Kang, M.; Mo, F.; Witmans, M.; Santiago, V.; Tablizo, M.A. Trends in Diagnosing Obstructive Sleep Apnea in Pediatrics. Children 2022, 9, 306.
- Huang, Y.-S.; Guilleminault, C. Pediatric Obstructive Sleep Apnea: Where Do We Stand? In Advances in Oto-Rhino-Laryngology; Lin, H.-C., Ed.; S. Karger AG: Basel, Switzerland, 2017; Volume 80, pp. 136–144. ISBN 978-3-318-06064-5.
- Pizarro, G.U.; Costa, E.L.d.B.; Pradella-Hallinan, M.; Meurer, A.T.d.O.; Moreira, G.A.; Fujita, R.R. Efficacy of Adenotonsillectomy in the Treatment of Obstructive Apnea in Children: A 2-Year Follow-Up. Int. J. Pediatr. Otorhinolaryngol. 2023, 166, 111462.
- Parmar, A.; Baker, A.; Narang, I. Positive Airway Pressure in Pediatric Obstructive Sleep Apnea. Paediatr. Respir. Rev. 2019, 31, 43–51.
- Machaalani, R.; Evans, C.A.; Waters, K.A. Objective Adherence to Positive Airway Pressure Therapy in an Australian Paediatric Cohort. Sleep Breath. 2016, 20, 1327–1336.
- Xiao, L.; Baker, A.; Voutsas, G.; Massicotte, C.; Wolter, N.E.; Propst, E.J.; Narang, I. Positional Device Therapy for the Treatment of Positional Obstructive Sleep Apnea in Children: A Pilot Study. Sleep Med. 2021, 85, 313–316.
- Tholen, K.; Meier, M.; Kloor, J.; Friedman, N. Persistent OSA in Obese Children: Does Body Position Matter? J. Clin. Sleep Med. 2021, 17, 227–232.
- Brockbank, J.C. Update on Pathophysiology and Treatment of Childhood Obstructive Sleep Apnea Syndrome. Paediatr. Respir. Rev. 2017, 24, 21–23.
- Andersen, I.G.; Holm, J.-C.; Homøe, P. Impact of Weight-Loss Management on Children and Adolescents with Obesity and Obstructive Sleep Apnea. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 57–62.
- Nixon, G.M.; Perrett, K.P. Limited Evidence for Anti-inflammatory Medications for Obstructive Sleep Apnoea in Children. J. Paediatr. Child Health 2021, 57, 2019–2021.
- Kuhle, S.; Urschitz, M.S. Anti-Inflammatory Medications for the Treatment of Pediatric Obstructive Sleep Apnea. Paediatr. Respir. Rev. 2020, 34, 35–36.
- Zhang, F.; Tian, Z.; Shu, Y.; Zou, B.; Yao, H.; Li, S.; Li, Q. Efficiency of Oro-Facial Myofunctional Therapy in Treating Obstructive Sleep Apnoea: A Meta-Analysis of Observational Studies. J. Oral Rehabil. 2022, 49, 734–745.
- Bandyopadhyay, A.; Kaneshiro, K.; Camacho, M. Effect of Myofunctional Therapy on Children with Obstructive Sleep Apnea: A Meta-Analysis. Sleep Med. 2020, 75, 210–217.
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376.




