1. Definition and Prevalence of Obstructive Sleep Apnea in Children
Sleep-disordered breathing (SDB) occurs as a result of upper airway (UA) dysfunction (snoring and/or increased respiratory effort). It ranges from snoring to obstructive sleep apnea (OSA), depending on the degree of intermittent UA obstruction [
1], and around 20% of children who snore have OSA [
2].
OSA is characterized by recurrent events of partial (hypopnea) or complete (apnea) obstructions in the UA, which disrupt normal oxygenation, ventilation, and sleep patterns [
1,
3,
4,
5]. OSA in children has a clear entity with profiles that are very different from adults in terms of etiology, clinical presentation, and consequences (
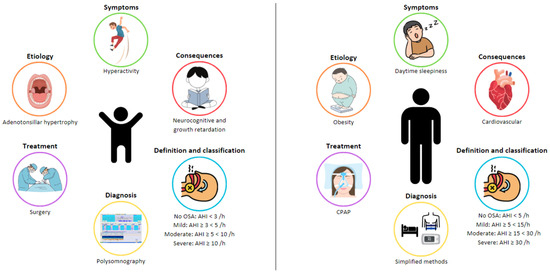
Figure 1). For this reason, a specific definition, diagnosis, and treatment approach is needed for this specific population.
Figure 1. Differences in obstructive sleep apnea (OSA) between adults and children.
OSA is a very frequent condition in children, with prevalence varying between 1 and 4% [
6]. Although there has been an effort to increase knowledge about this entity in childhood, there is less scientific evidence than in adults. Different guidelines establish the definition of SDB and OSA in children, although the criteria are diverse and lack recent updates. The classification of OSA severity in children, through the apnea/hypopnea index-(AHI) (number of respiratory events per hour of sleep) obtained from sleep studies, is the most-commonly used parameter. Generally, an AHI of 1–3/h is accepted as the normal cutoff line for the diagnosis of OSA and is classified as follows: mild OSA if the AHI < 5/h, moderate OSA when the AHI is between 5 and 10/h, and severe OSA when the AHI > 10/h [
1,
3,
5,
7]. However, these criteria can vary depending on the guidelines, considering factors such as age, additional comorbidities, and other polysomnographic variables (presence and length of oxygen desaturations, degree of hypoventilation, sleep fragmentation, and decreased total sleep time) [
7,
8].
2. Etiology of OSA in Children
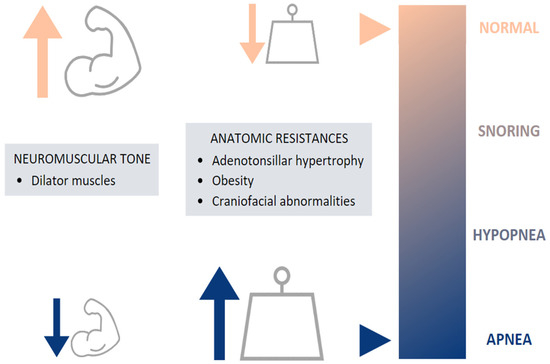
The etiology of childhood OSA is multifactorial, involving many risk factors, which can increase UA narrowing and collapsibility and which may contribute to the pathogenesis of OSA [
8,
9,
10]. This includes both anatomical and neuromuscular disturbances, leading to increased airway resistance and preventing the normal function of the dilator muscles, respectively [
5] (
Figure 2).
Figure 2. Etiology of pediatric OSA.
The most-common risk factor is adenotonsillar hypertrophy, reaching the peak of development between 2 and 8 years [
11], coinciding with the onset of OSA [
12]. Nevertheless, some studies have shown a weak or no correlation between the size of the tonsils and adenoids and the severity of pediatric OSA [
13,
14]. Craniofacial abnormalities can also be a cause of UA narrowing: alterations of the size, position, and geometry of the mandible and the tongue [
10]. These anatomical features are often found in children with craniofacial syndromes, achondroplasia, trisomy 21, Beckwith–Wiedemann syndrome, Chiari malformation, and mucopolysaccharidoses [
8].
Besides anatomic factors, obesity has also been suggested as a contributor to OSA. Obese children represent a special risk factor, as the prevalence of childhood obesity is progressively increasing (5.6% in girls and 7.8% in boys) [
15], also leading to an increase in the prevalence of obesity-associated morbidities including OSA [
10,
11,
16]. This relationship is bidirectional, as OSA is known to worsen weight loss and overweight [
17,
18].
3. Symptoms of OSA in Children
The symptoms are classically divided into nocturnal and diurnal (
Table 1). Nocturnal symptoms include snoring, witnessed apneas, gasping, oral breathing, paradoxical thoracic movements, nightmares, restless sleep, and nocturnal enuresis. Snoring is the most-common symptom, along with oral breathing. This population can also present disturbed sleep with frequent changes of position, unusual sleep positions (neck hyperextension), and nightmares [
9,
19]. Enuresis is another frequent symptom in OSA children related to an altered arousal response and sleep fragmentation, often being resolved when OSA is adequately treated [
20].
Table 1. Symptoms of OSA in children.
Related to daytime symptoms, a relationship between OSA and behavioral disorders (irritability, aggressiveness, and depression), neurocognitive disorders (difficulty concentrating/learning difficulties and inattention), mood instability, and excessive daytime sleepiness has been demonstrated [
5,
10,
21,
22,
23].
4. Consequences
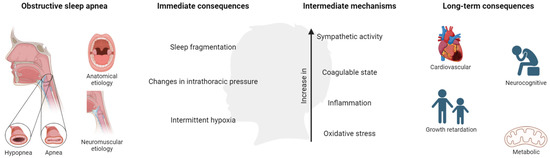
OSA in children is associated with a number of adverse morbidities, presented as behavioral and neurocognitive disorders, growth retardation, cardiovascular (CV) diseases, and metabolic consequences, producing a negative impact on quality of life. These consequences are derived from the presence of continuous episodes of hypoxia/resaturation, sleep fragmentation, and/or changes in the intrathoracic pressure. These immediate consequences develop a cascade of intermediate mechanisms, mainly alterations in sympathetic activity, coagulation, inflammation, and oxidative stress (Figure 3).
Figure 3. Mechanisms and consequences of OSA in children. Created with BioRender.com. This population is characterized by poor academic performance showing a reduction in memory capacities and difficulties in learning and attention (especially in specific areas such as mathematics, science, reading, and spelling) [
24,
25,
26], which could be associated with hyperactive behavior during the day.
The effects on growth are probably related to factors such as increased energy consumption and reduced production of growth hormone, whose secretion is characterized by wide and frequent peaks during sleep [
9]. These adverse results may be recovered after OSA treatment, as suggested by different studies [
27,
28,
29].
In the CV sphere, alterations in the autonomic nervous system, vasomotor tone, systemic inflammation, and atherogenesis associated with OSA are likely to induce functional disruption of the endothelium [
30]. In addition, many biomarkers have been evaluated to identify this vascular damage, the C-reactive protein (CRP) being the most-studied marker. This inflammatory indicator is increased in children with OSA, with a recent study indicating that it could be reversed after treatment [
31]. It has been reported that children with OSA have increased systolic and diastolic blood pressure (BP), increased BP variability, and decreased BP dipping during sleep. Observing the BP of children with OSA is essential to identifying those at risk for developing clinically significant elevated BP in adulthood [
32]. There is an independent effect of OSA on cardiopulmonary function, which improves after the disorder is adequately treated [
33,
34,
35]. Finally, these children may develop an early metabolic syndrome [
10,
36], this risk being six-times higher than in healthy subjects in adolescents with OSA [
37]. A brief literature search of recent evidence in these spheres is described in
Table 2.
Table 2. Consequences of OSA in children.
New evidence regarding untreated pediatric OSA’s significant long-term morbidities affecting different organs and systems is necessary [
41]. Therefore, in order to minimize the deleterious consequences related to OSA, early correct diagnosis and treatment management are mandatory. The strongest evidence shows that this population may have a significant impact on CV health in childhood and later in adulthood [
30]. Tools to identify children at risk and treatment prevention indication are needed.
5. Diagnosis
The diagnosis of OSA in the pediatric population differs according to the different clinical guidelines and is described according to the Spanish [
5], European [
1], and American [
3] guidelines in
Table 3.
Table 3. Diagnosis and management of OSA in children.
Although medical history and physical examination are useful to screen and determine which patients are suspected of having OSA, the sensitivity and specificity are scarce. Thus, objective sleep tests are needed. The gold standard is overnight, attended, in-laboratory polysomnography (PSG), a complex test that records neurophysiological and cardiorespiratory variables. The American Academy of Sleep Medicine (AASM) in 2007 described the rules for the scoring of sleep and respiratory events in PSG recordings [
4], last upgraded in February 2023, these being different for children than adults.
However, PSG may not be readily available, so alternative diagnostic tests can be performed: daytime nap PSG, ambulatory PSG, respiratory polygraphy (RP), nocturnal oximetry, the Pediatric Sleep Questionnaire, or nocturnal video recording. The complexity and limitations of PSG entail an increase in the development and validation of alternative methods for the diagnosis of OSA in children [
42]. As an example, the European guideline accepts hospital RP as a valid alternative for the diagnosis of OSA in children and is considered an adequate screening technique when PSG is not available (
Table 3).
6. Treatment
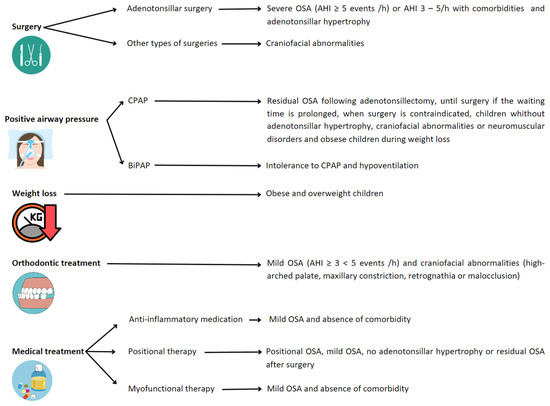
The goal of OSA treatment is complete resolution of SDB. This may require combining strategies (
Figure 4), although the first-line treatment for OSA in children is adenotonsillar surgery [
5] when adenotonsillar hypertrophy is present. Nonetheless, in the recent past, this treatment has been questioned. Recent publications have shown that the use of adenotonsillectomy in pediatric OSA patients may have variable results, reaching an AHI of 1 or less in about 50–70% of cases, but its efficacy decreases with risk factors such as age (<7 years), severe disease, chronic asthma or obesity. Persistent disease is present in 20–75% of children, with more than half having habitual snoring [
7,
43,
44]. In addition, other surgical procedures may be performed in selected cases, such as septoplasty, uvulopharyngopalatoplasty, epiglottoplasty, glossopexy, and maxillomandibular surgery.
Figure 4. Recommended treatments depending on conditions leading to OSA in children. Abbreviations: OSA: obstructive sleep apnea; AHI: apnea/hypopnea index; CPAP: continuous positive airway pressure; BiPAP: bi-level positive airway pressure.
For those children with residual OSA following adenotonsillectomy or those in whom surgery is contraindicated or without adenotonsillar hypertrophy, positive airway pressure (PAP) therapies can be an effective treatment. The two types of PAP therapies prescribed in children to treat OSA are continuous positive airway pressure (CPAP) and bi-level positive airway pressure (Bi-PAP) [
45]. CPAP is the most-commonly used PAP therapy, also recommended in children with craniofacial abnormalities or neuromuscular disorders [
1,
3,
5]. The use of BiPAP is for patients intolerant to CPAP to treat nocturnal hypoventilation [
46].
Positional therapy, as an alternative treatment, has been widely studied and relatively implemented in adults for the management of positional OSA. Positional OSA is defined when, spending more than 20% of sleep time in the supine position, the AHI in the supine position is at least double that in the non-supine position. This definition has not been adapted to the pediatric population and is, therefore, assumed in the child. In adults, positional therapy is incorporated in cases of mild–moderate OSA of positional origin and in those with severe OSA in order to lower CPAP pressure or when there is intolerance to first-line treatment. However, the indications in the pediatric population are not clearly established, and the scientific evidence is scarce. In this sense, it seems that children without tonsillar hypertrophy or with residual OSA could benefit from it, mainly in cases of obesity [
47,
48]. Therefore, randomized studies are necessary to establish the efficacy and indications of this type of therapy in the pediatric patient.
Weight management, orthodontic treatment, or medical therapy are offered as an alternative to surgery, especially in children with mild OSA [
7,
8,
49] or when surgery is not indicated or contraindicated. There are data supporting that weight loss, if the child is overweight or obese, can improve OSA (hence, proposed to be considered first-line treatment in this population) [
50]. Rapid maxillary expansion or orthodontic appliances are used to widen the palate and cause flattening of the palatal arch. On the other hand, medical therapies such as anti-inflammatory medications (nasal corticosteroid and/or oral montelukast) can also be used. There is little evidence about anti-inflammatory therapies in children. The results of randomized clinical trials evaluating the efficacy of intranasal corticosteroids for the treatment of OSA are not conclusive. Montelukast has short-term beneficial treatment effects for OSA in healthy, non-obese, surgically untreated children in terms of reducing the AHI, but the clinical relevance remains unclear [
51,
52]. Finally, myofunctional therapy has been accepted as a non-invasive treatment for OSA in children, as it may improve the AHI and oxygen saturation, at least after tonsillectomy or as an adjunct OSA treatment [
53,
54].
It is worth mentioning that obstructive SDB can be resolved spontaneously, particularly in children with mild OSA and adenotonsillar hypertrophy. Improvements may be due to the regression of lymphoid tissue or growth of the airway [
55].
In summary, current data on the management of pediatric patients with OSA around the world, presented in this research, manifest important discrepancies and the need to be updated and homogenized. An agreed upon definition is needed with specific cutoff points to establish the diagnosis and levels of severity based on the associated risk and comorbidities. Clear diagnostic management algorithms must be settled upon in which it is defined when the simplified methods are useful in pediatric patients, in order to avoid underdiagnosis. It is necessary to identify prognostic markers that set up cutoff points for treatment indications based on objective impact. In addition, new metrics that better evaluate the disease could lead to the establishment of new protocols that improve the treatment management of the child.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11061708