
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elsa Anes | -- | 1631 | 2023-06-20 15:43:48 | | | |
| 2 | Conner Chen | Meta information modification | 1631 | 2023-06-25 05:00:45 | | |
Video Upload Options
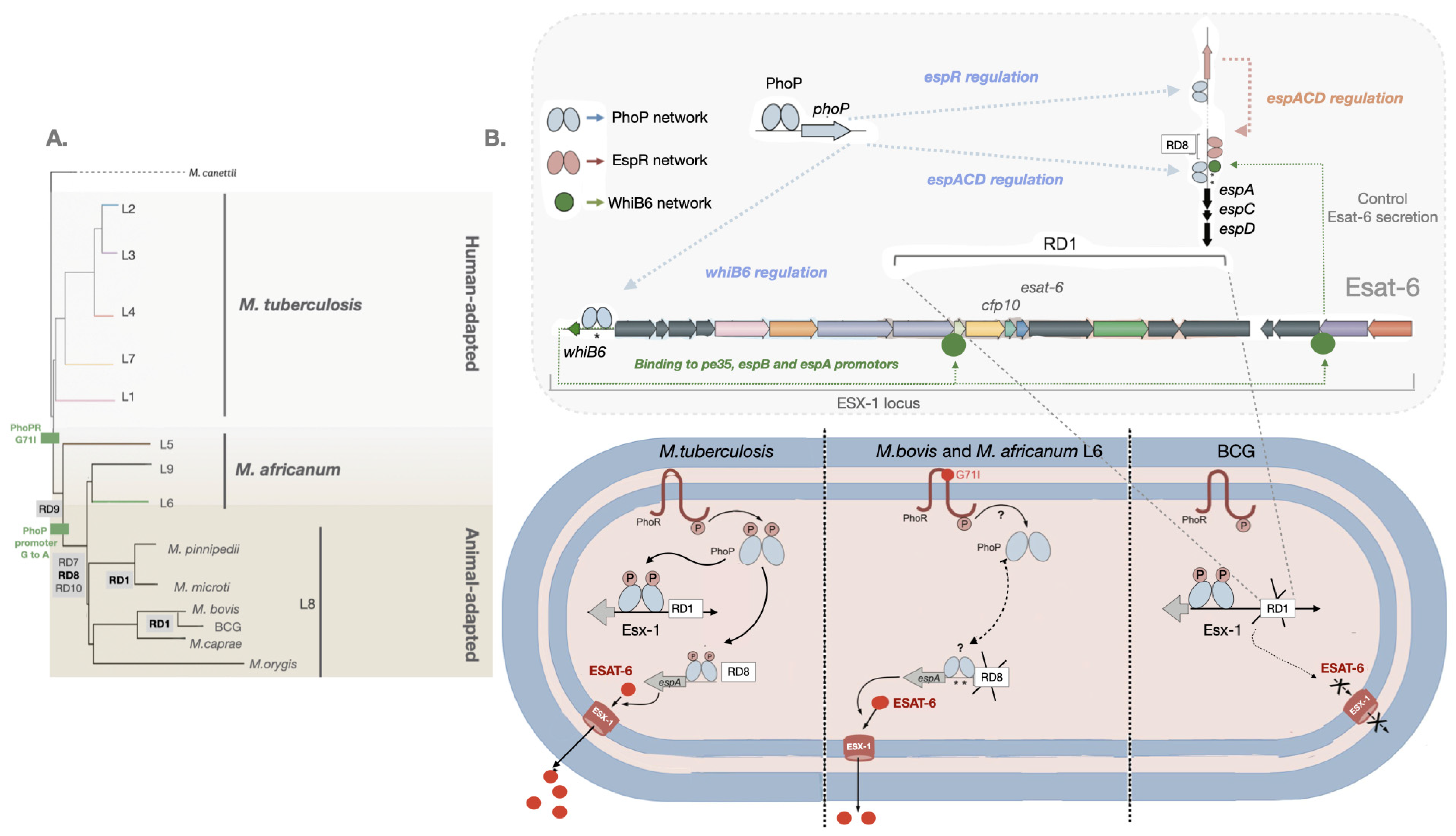
Mycobacterium tuberculosis (Mtb) virulence relies on its ability to manipulate host macrophages, where it establishes intracellular niches to cross mucosal barriers and avoid pathogen destruction. First, Mtb subverts the endocytic pathway, preventing phagolysosome fusion and proteolytic digestion. Second, it activates innate immune responses to induce its transmigration into the lung parenchyma. There, infected macrophages attract more permissive cells, expanding intracellular niches. Mtb induces the adaptive responses that stimulate its containment and encourage a long life inside granulomas. Finally, the pathogen induces necrotic cell death in macrophages, granuloma destruction, and lung cavitation for transmission. Common to all these events is the major virulence factor: the “early secreted antigenic target of 6 kDa” (ESAT-6, also called EsxA). The loss or gain of mycobacterial virulence is closely linked to the ability of mycobacteria to produce and secrete ESAT-6, and the extension of virulence is correlated with the amount of protein secreted. ESAT-6 secretion from the bacilli requires both the expression of the esx-1 locus for the type VII secretion apparatus and the transcription of both the ESAT-6 gene (esxA) and the culture filtrate protein 10 (CFP-10) gene (esx-B) contained in the RD1 region of the genome. In addition, it requires the protein EspA, which is not encoded in the esx-1 locus but in the extended espACD operon adjacent to RD8. All species and strains deleted in the esx-1 locus, the internal RD1 region, or the esx-1 extended locus espACD exhibit an attenuated phenotype. Mutants with deletions on ESX-1 of Mtb are attenuated in virulence, translating into reduced survival of mycobacteria in cultured macrophages or in experimental animal models of TB. Curiously, the saprophyte species M. smegmatis (Ms) also encodes for an ESX-1 apparatus; however, it does not appear to confer Ms virulence capabilities, as demonstrated by its inability to survive in human macrophages or in amoeba in the environment. Predatory amoeba may have contributed to the evolutionary pressure that selected mycobacterial pathogens for intracellular survival.
1. Introduction
2. Virulence Evolution among MTBC
References
- Perrin, P. Human and tuberculosis co-evolution: An integrative view. Tuberculosis 2015, 95, S112–S116.
- Wilson, L.G. Commentary: Medicine, population, and tuberculosis. Int. J. Epidemiol. 2005, 34, 521–524.
- WHO. Global Tuberculosis Report 2022 Factsheet. Available online: https://www.who.int/publications/m/item/global-tuberculosis-report-2022-factsheet (accessed on 20 April 2023).
- Chen, X.; Hu, T.Y. Strategies for advanced personalized tuberculosis diagnosis: Current technologies and clinical approaches. Precis. Clin. Med. 2021, 4, 35–44.
- Behr, M.A.; Kaufmann, E.; Duffin, J.; Edelstein, P.H.; Ramakrishnan, L. Latent Tuberculosis: Two Centuries of Confusion. Am. J. Respir. Crit. Care Med. 2021, 204, 142–148.
- Carranza, C.; Pedraza-Sanchez, S.; de Oyarzabal-Mendez, E.; Torres, M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Front. Immunol. 2020, 11, 2006.
- Dheda, K.; Barry, C.E., 3rd; Maartens, G. Tuberculosis. Lancet 2016, 387, 1211–1226.
- Dheda, K.; Gumbo, T.; Maartens, G.; Dooley, K.E.; McNerney, R.; Murray, M.; Furin, J.; Nardell, E.A.; London, L.; Lessem, E.; et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 2017, 5, 291–360.
- Cambier, C.J.; Falkow, S.; Ramakrishnan, L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell 2014, 159, 1497–1509.
- Azevedo-Pereira, J.M.; Pires, D.; Calado, M.; Mandal, M.; Santos-Costa, Q.; Anes, E. HIV/Mtb Co-Infection: From the Amplification of Disease Pathogenesis to an “Emerging Syndemic”. Microorganisms 2023, 11, 853.
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855.
- Blaser, M.J.; Kirschner, D. The equilibria that allow bacterial persistence in human hosts. Nature 2007, 449, 843–849.
- Evans, J.T.; Smith, E.G.; Banerjee, A.; Smith, R.M.M.; Dale, J.; Innes, J.A.; Hunt, D.; Tweddell, A.; Wood, A.; Anderson, C.; et al. Cluster of human tuberculosis caused by Mycobacterium bovis: Evidence for person-to-person transmission in the UK. Lancet 2007, 369, 1270–1276.
- Grange, J.M. Mycobacterium bovis infection in human beings. Tuberculosis 2001, 81, 71–77.
- Thoen, C.O.; LoBue, P.A. Mycobacterium bovis tuberculosis: Forgotten, but not gone. Lancet 2007, 369, 1236–1238.
- Torres-Gonzalez, P.; Cervera-Hernandez, M.E.; Martinez-Gamboa, A.; Garcia-Garcia, L.; Cruz-Hervert, L.P.; Bobadilla-del Valle, M.; Ponce-de Leon, A.; Sifuentes-Osornio, J. Human tuberculosis caused by Mycobacterium bovis: A retrospective comparison with Mycobacterium tuberculosis in a Mexican tertiary care centre, 2000–2015. BMC Infect. Dis. 2016, 16, 657.
- Wilkins, E.G.; Griffiths, R.J.; Roberts, C. Pulmonary tuberculosis due to Mycobacterium bovis. Thorax 1986, 41, 685.
- de Jong, B.C.; Hill, P.C.; Aiken, A.; Awine, T.; Martin, A.; Adetifa, I.M.; Jackson-Sillah, D.J.; Fox, A.; Kathryn, D.; Gagneux, S.; et al. Progression to Active Tuberculosis, but Not Transmission, Varies by Mycobacterium tuberculosis Lineage in The Gambia. J. Infect. Dis. 2008, 198, 1037–1043.
- Mostowy, S.; Onipede, A.; Gagneux, S.; Niemann, S.; Kremer, K.; Desmond Edward, P.; Kato-Maeda, M.; Behr, M. Genomic Analysis Distinguishes Mycobacterium africanum. J. Clin. Microbiol. 2004, 42, 3594–3599.
- Silva, M.L.; Cá, B.; Osório, N.S.; Rodrigues, P.N.S.; Maceiras, A.R.; Saraiva, M. Tuberculosis caused by Mycobacterium africanum: Knowns and unknowns. PLoS Pathog. 2022, 18, e1010490.
- de Jong, B.C.; Antonio, M.; Gagneux, S. Mycobacterium africanum—Review of an Important Cause of Human Tuberculosis in West Africa. PLoS Negl. Trop. Dis. 2010, 4, e744.
- Coscolla, M.; Gagneux, S.; Menardo, F.; Loiseau, C.; Ruiz-Rodriguez, P.; Borrell, S.; Otchere, I.D.; Asante-Poku, A.; Asare, P.; Sánchez-Busó, L.; et al. Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Microb. Genom. 2021, 7, 000477.
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213.
- Müller, B.; Dürr, S.; Alonso, S.; Hattendorf, J.; Laisse, C.J.M.; Parsons, S.D.C.; van Helden, P.; Zinsstag, J. Zoonotic Mycobacterium bovis–induced Tuberculosis in Humans. Emerg. Infect. Dis. 2013, 19, 899–908.
- Borham, M.; Oreiby, A.; El-Gedawy, A.; Hegazy, Y.; Khalifa, H.O.; Al-Gaabary, M.; Matsumoto, T. Review on Bovine Tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species. Pathogens 2022, 11, 715.
- Prodinger, W.M.; Indra, A.; Koksalan, O.K.; Kilicaslan, Z.; Richter, E. Mycobacterium caprae infection in humans. Expert Rev. Anti-Infect. Ther. 2014, 12, 1501–1513.
- Tagliapietra, V.; Boniotti, M.B.; Mangeli, A.; Karaman, I.; Alborali, G.; Chiari, M.; D’Incau, M.; Zanoni, M.; Rizzoli, A.; Pacciarini, M.L. Mycobacterium microti at the Environment and Wildlife Interface. Microorganisms 2021, 9, 2084.
- Fabre, M.; Hauck, Y.; Soler, C.; Koeck, J.-L.; van Ingen, J.; van Soolingen, D.; Vergnaud, G.; Pourcel, C. Molecular characteristics of “Mycobacterium canettii” the smooth Mycobacterium tuberculosis bacilli. Infect. Genet. Evol. 2010, 10, 1165–1173.
- Gonzalo-Asensio, J.; Malaga, W.; Pawlik, A.; Astarie-Dequeker, C.; Passemar, C.; Moreau, F.; Laval, F.; Daffé, M.; Martin, C.; Brosch, R.; et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl. Acad. Sci. USA 2014, 111, 11491–11496.
- Riojas, M.A.; McGough, K.J.; Rider-Riojas, C.J.; Rastogi, N.; Hazbón, M.H. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int. J. Syst. Evol. Microbiol. 2018, 68, 324–332.
- Lombard, J.E.; Patton, E.A.; Gibbons-Burgener, S.N.; Klos, R.F.; Tans-Kersten, J.L.; Carlson, B.W.; Keller, S.J.; Pritschet, D.J.; Rollo, S.; Dutcher, T.V.; et al. Human-to-Cattle Mycobacterium tuberculosis Complex Transmission in the United States. Front. Vet. Sci. 2021, 8, 691192.
- WHO. Roadmap for Zoonotic Tuberculosis. Available online: https://www.who.int/publications/i/item/9789241513043 (accessed on 29 May 2023).
- Wolfe, N.D.; Dunavan, C.P.; Diamond, J. Origins of major human infectious diseases. Nature 2007, 447, 279–283.
- Comas, I.; Coscolla, M.; Luo, T.; Borrell, S.; Holt, K.E.; Kato-Maeda, M.; Parkhill, J.; Malla, B.; Berg, S.; Thwaites, G.; et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 2013, 45, 1176–1182.
- Urbanowski, M.E.; Ordonez, A.A.; Ruiz-Bedoya, C.A.; Jain, S.K.; Bishai, W.R. Cavitary tuberculosis: The gateway of disease transmission. Lancet Infect. Dis. 2020, 20, e117–e128.
- Brites, D.; Gagneux, S. Old and new selective pressures on Mycobacterium tuberculosis. Infect. Genet. Evol. 2012, 12, 678–685.
- Alteri, C.J.; Rios-Sarabia, N.; De la Cruz, M.A.; González-y-Merchand, J.A.; Soria-Bustos, J.; Maldonado-Bernal, C.; Cedillo, M.L.; Yáñez-Santos, J.A.; Martínez-Laguna, Y.; Torres, J.; et al. The Flp type IV pilus operon of Mycobacterium tuberculosis is expressed upon interaction with macrophages and alveolar epithelial cells. Front. Cell. Infect. Microbiol. 2022, 12, 1382.
- Ramsugit, S.; Pillay, M. Pili of Mycobacterium tuberculosis: Current knowledge and future prospects. Arch. Microbiol. 2015, 197, 737–744.
- Kalscheuer, R.; Palacios, A.; Anso, I.; Cifuente, J.; Anguita, J.; Jacobs, W.R., Jr.; Guerin, M.E.; Prados-Rosales, R. The Mycobacterium tuberculosis capsule: A cell structure with key implications in pathogenesis. Biochem. J. 2019, 476, 1995–2016.
- Anes, E.; Pires, D.; Mandal, M.; Azevedo-Pereira, J.M. Spatial localization of cathepsins: Implications in immune activation and resolution during infections. Front. Immunol. 2022, 13, 955407.
- Armstrong, J.A.; Hart, P.D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 1975, 142, 1–16.
- Pires, D.; Bernard, E.M.; Pombo, J.P.; Carmo, N.; Fialho, C.; Gutierrez, M.G.; Bettencourt, P.; Anes, E. Mycobacterium tuberculosis Modulates miR-106b-5p to Control Cathepsin S Expression Resulting in Higher Pathogen Survival and Poor T-Cell Activation. Front. Immunol. 2017, 8, 1819.
- Pires, D.; Calado, M.; Velez, T.; Mandal, M.; Catalão, M.J.; Neyrolles, O.; Lugo-Villarino, G.; Vérollet, C.; Azevedo-Pereira, J.M.; Anes, E. Modulation of Cystatin C in Human Macrophages Improves Anti-Mycobacterial Immune Responses to Mycobacterium tuberculosis Infection and Coinfection With HIV. Front. Immunol. 2021, 12, 742822.
- Pires, D.; Mandal, M.; Pinho, J.; Catalão, M.J.; Almeida, A.J.; Azevedo-Pereira, J.M.; Gaspar, M.M.; Anes, E. Liposomal Delivery of Saquinavir to Macrophages Overcomes Cathepsin Blockade by Mycobacterium tuberculosis and Helps Control the Phagosomal Replicative Niches. Int. J. Mol. Sci. 2023, 24, 1142.
- Pires, D.; Marques, J.; Pombo, J.P.; Carmo, N.; Bettencourt, P.; Neyrolles, O.; Lugo-Villarino, G.; Anes, E. Role of Cathepsins in Mycobacterium tuberculosis Survival in Human Macrophages. Sci. Rep. 2016, 6, 32247.
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.e4.
- Mishra, B.B.; Moura-Alves, P.; Sonawane, A.; Hacohen, N.; Griffiths, G.; Moita, L.F.; Anes, E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 2010, 12, 1046–1063.
- Aguilo, J.I.; Alonso, H.; Uranga, S.; Marinova, D.; Arbués, A.; de Martino, A.; Anel, A.; Monzon, M.; Badiola, J.; Pardo, J.; et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell. Microbiol. 2013, 15, 1994–2005.
- Dallenga, T.; Repnik, U.; Corleis, B.; Eich, J.; Reimer, R.; Griffiths, G.W.; Schaible, U.E. M. tuberculosis-Induced Necrosis of Infected Neutrophils Promotes Bacterial Growth Following Phagocytosis by Macrophages. Cell Host Microbe 2017, 22, 519–530.e3.
- Derrick, S.C.; Morris, S.L. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell. Microbiol. 2007, 9, 1547–1555.
- Anes, E.; Azevedo-Pereira, J.M.; Pires, D. Cathepsins and Their Endogenous Inhibitors in Host Defense During Mycobacterium tuberculosis and HIV Infection. Front. Immunol. 2021, 12, 726984.
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49.
- Pagán, A.J.; Ramakrishnan, L. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb. Perspect. Med. 2015, 5, a018499.
- Wolf, A.J.; Linas, B.; Trevejo-Nuñez, G.J.; Kincaid, E.; Tamura, T.; Takatsu, K.; Ernst, J.D. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo1. J. Immunol. 2007, 179, 2509–2519.
- Mishra, B.B.; Rathinam, V.A.K.; Martens, G.W.; Martinot, A.J.; Kornfeld, H.; Fitzgerald, K.A.; Sassetti, C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome–dependent processing of IL-1β. Nat. Immunol. 2013, 14, 52–60.
- Wong, K.-W.; Jacobs Jr, W.R. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 2011, 13, 1371–1384.
- Brosch, R.; Gordon, S.V.; Marmiesse, M.; Brodin, P.; Buchrieser, C.; Eiglmeier, K.; Garnier, T.; Gutierrez, C.; Hewinson, G.; Kremer, K.; et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 2002, 99, 3684–3689.
- Brosch, R.; Gordon, S.V.; Garnier, T.; Eiglmeier, K.; Frigui, W.; Valenti, P.; Dos Santos, S.; Duthoy, S.; Lacroix, C.; Garcia-Pelayo, C.; et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. USA 2007, 104, 5596–5601.
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544.
- Marinova, D.; Gonzalo-Asensio, J.; Aguilo, N.; Martin, C. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev. Vaccines 2017, 16, 565–576.
- Smith, N.H.; Hewinson, R.G.; Kremer, K.; Brosch, R.; Gordon, S.V. Myths and misconceptions: The origin and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2009, 7, 537–544.
- Broset, E.; Martín, C.; Gonzalo-Asensio, J. Evolutionary Landscape of the Mycobacterium tuberculosis Complex from the Viewpoint of PhoPR: Implications for Virulence Regulation and Application to Vaccine Development. Mbio 2015, 6, e01289-15.
- Frigui, W.; Bottai, D.; Majlessi, L.; Monot, M.; Josselin, E.; Brodin, P.; Garnier, T.; Gicquel, B.; Martin, C.; Leclerc, C.; et al. Control of M. tuberculosis ESAT-6 Secretion and Specific T Cell Recognition by PhoP. PLoS Pathog. 2008, 4, e33.
- Pérez, E.; Samper, S.; Bordas, Y.; Guilhot, C.; Gicquel, B.; Martín, C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 2001, 41, 179–187.
- Mahairas, G.G.; Sabo, P.J.; Hickey, M.J.; Singh, D.C.; Stover, C.K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 1996, 178, 1274–1282.
- Pym, A.S.; Brodin, P.; Brosch, R.; Huerre, M.; Cole, S.T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 2002, 46, 709–717.
- de Jong, B.C.; Hill, P.C.; Brookes, R.H.; Gagneux, S.; Jeffries, D.J.; Otu, J.K.; Donkor, S.A.; Fox, A.; McAdam, K.P.W.J.; Small, P.M.; et al. Mycobacterium africanum Elicits an Attenuated T Cell Response to Early Secreted Antigenic Target, 6 kDa, in Patients with Tuberculosis and Their Household Contacts. J. Infect. Dis. 2006, 193, 1279–1286.
- Gonzalo-Asensio, J.; Pérez, I.; Aguiló, N.; Uranga, S.; Picó, A.; Lampreave, C.; Cebollada, A.; Otal, I.; Samper, S.; Martín, C. New insights into the transposition mechanisms of IS6110 and its dynamic distribution between Mycobacterium tuberculosis Complex lineages. PLoS Genet. 2018, 14, e1007282.
- Soto, C.Y.; Menendez, M.C.; Perez, E.; Samper, S.; Gomez, A.B.; Garcia, M.J.; Martin, C. IS6110 Mediates Increased Transcription of the phoP Virulence Gene in a Multidrug-Resistant Clinical Isolate Responsible for Tuberculosis Outbreaks. J. Clin. Microbiol. 2004, 42, 212–219.
- Abdallah, A.M.; Gey van Pittius, N.C.; DiGiuseppe Champion, P.A.; Cox, J.; Luirink, J.; Vandenbroucke-Grauls, C.M.J.E.; Appelmelk, B.J.; Bitter, W. Type VII secretion—Mycobacteria show the way. Nat. Rev. Microbiol. 2007, 5, 883–891.
- Gröschel, M.I.; Sayes, F.; Simeone, R.; Majlessi, L.; Brosch, R. ESX secretion systems: Mycobacterial evolution to counter host immunity. Nat. Rev. Microbiol. 2016, 14, 677–691.




