
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jan-Peter Hildebrandt | -- | 2295 | 2023-06-19 17:44:08 | | | |
| 2 | Fanny Huang | Meta information modification | 2295 | 2023-06-26 05:25:43 | | |
Video Upload Options
Hyperplasia and hypertrophy or their counterparts, hypoplasia and hypotrophy, are elements of adjusting organ size and function in animals according to needs under altered environmental conditions. As such processes are costly in terms of energy and biomaterials, it is assumed that they are beneficial for the survival of the individual. The ability of animals to perform such adjustments and their limitations in scope are considered to be adaptive genetic traits which enable individual animals to survive regularly occurring changes in environmental conditions in their habitats as long as such changes stay within critical limits. The restructuring of mono-functional glands in ducklings which serve the animals to get rid of excess amounts of ingested salt from the body is presented as an example of complex plastic changes in organ structure. Phenotypic adjustments in these salt glands encompass processes which are reversible when environmental conditions switch back to the original state (‘phenotypic elasticity’) as well as irreversible ones (‘phenotypic plasticity’ in the narrow sense). As more information on genomes or transcriptomes of non-model animal species becomes available these days, researchers will better understand the biological significance of such phenotypic adjustments in animals in their natural environments and the underlying molecular mechanisms.
1. Introduction
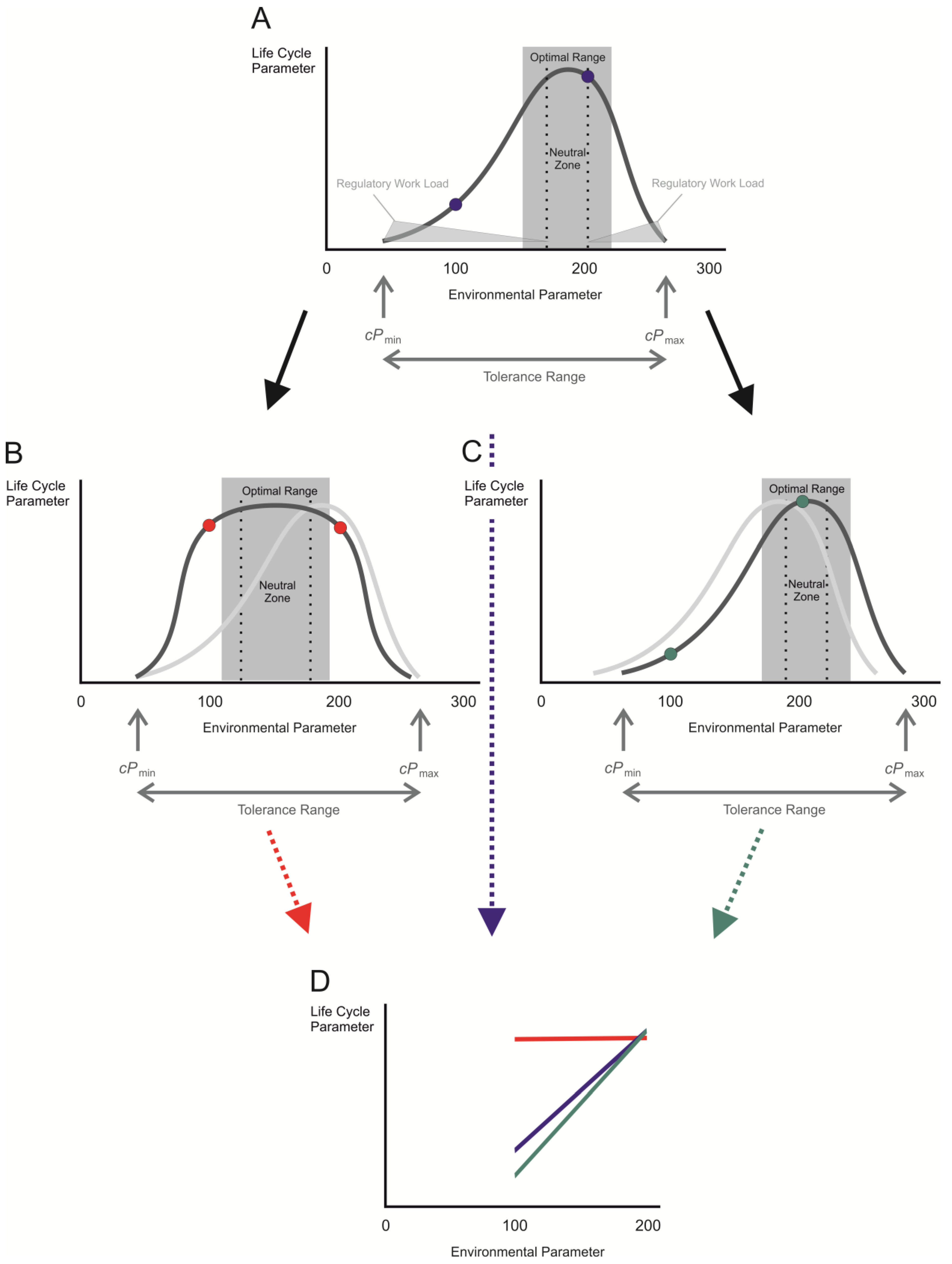
2. Measure for Plasticity in Response to Environmental Change: The Reaction Norm
3. Acclimation
4. Reversibility of Plastic Changes
References
- Pigliucci, M.; Schlichting, C.D.; Jones, C.S.; Schwenk, K. Developmental reaction norms: The interactions among allometry, ontogeny and plasticity. Plant Species Biol. 1996, 11, 69–85.
- Weinkove, D.; Leevers, S.J. The genetic control of organ growth: Insights from Drosophila. Curr. Opin. Genet. Dev. 2000, 10, 75–80.
- Ferenci, T.; Maharjan, R. Mutational heterogeneity: A key ingredient of bet-hedging and evolutionary divergence? Bioessays 2015, 37, 123–130.
- Hoban, S.; Kelley, J.L.; Lotterhos, K.E.; Antolin, M.F.; Bradburd, G.; Lowry, D.B.; Poss, M.L.; Reed, L.K.; Storfer, A.; Whitlock, M.C. Finding the genomic basis of local adaptation: Pitfalls, practical solutions, and future directions. Am. Nat. 2016, 188, 379–397.
- Charmantier, A.; Garant, D. Environmental quality and evolutionary potential: Lessons from wild populations. Proc. Biol. Sci. 2005, 272, 1415–1425.
- Woods, H.A. Mosaic physiology from developmental noise: Within-organism physiological diversity as an alternative to phenotypic plasticity and phenotypic flexibility. J. Exp. Biol. 2014, 217, 35–45.
- de Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common garden experiments in the genomic era: New perspectives and opportunities. Heredity 2016, 116, 249–254.
- Tejedor, J.R.; Fraga, M.F. Interindividual epigenetic variability: Sound or noise? Bioessays 2017, 39, 1700055.
- Cossins, A.; Fraser, J.; Hughes, M.; Gracey, A. Post-genomic approaches to understanding the mechanisms of environmentally induced phenotypic plasticity. J. Exp. Biol. 2006, 209, 2328–2336.
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367.
- Fusco, G.; Minelli, A. Phenotypic plasticity in development and evolution: Facts and concepts. Philos. Trans. R. Soc. B 2010, 365, 547–556.
- Beaman, J.E.; White, C.R.; Seebacher, F. Evolution of plasticity: Mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 2016, 31, 237–249.
- Chevin, L.-M.; Collins, S.; Lefèvre, F. Phenotypic plasticity and evolutionary demographic responses to climate change: Taking theory out to the field. Funct. Ecol. 2013, 27, 967–979.
- Minelli, A.; Fusco, G. Developmental plasticity and the evolution of animal complex life cycles. Philos. Trans. R. Soc. B 2010, 365, 631–640.
- Moczek, A.P.; Sultan, S.; Foster, S.; Ledon-Rettig, C.; Dworkin, I.; Nijhout, H.F.; Abouheif, E.; Pfennig, D.W. The role of developmental plasticity in evolutionary innovation. Proc. Biol. Sci. 2011, 278, 2705–2713.
- Peirson, B.R.E. Plasticity, stability, and yield: The origins of Anthony David Bradshaw’s model of adaptive phenotypic plasticity. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2015, 50, 51–66.
- Pfennig, D.W.; McGee, M. Resource polyphenism increases species richness: A test of the hypothesis. Philos. Trans. R. Soc. B 2010, 365, 577–591.
- Schlichting, C.D.; Smith, H. Phenotypic plasticity: Linking molecular mechanisms with evolutionary outcomes. Evol. Ecol. 2002, 16, 189–211.
- Sommer, R.J. Phenotypic plasticity: From theory and genetics to current and future challenges. Genetics 2020, 215, 1–13.
- Joschinski, J.; Bonte, D. Transgenerational plasticity and bet-hedging: A framework for reaction norm evolution. Front. Ecol. Evol. 2020, 8, 517183.
- Waldvogel, A.-M.; Feldmeyer, B.; Rolshausen, G.; Exposito-Alonso, M.; Rellstab, C.; Kofler, R.; Mock, T.; Schmid, K.; Schmitt, I.; Bataillon, T.; et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 2020, 4, 4–18.
- Leroi, A.M.; Bennett, A.F.; Lenski, R.E. Temperature acclimation and competitive fitness: An experimental test of the beneficial acclimation assumption. Proc. Natl. Acad. Sci. USA 1994, 91, 1917–1921.
- Palacio-Lopez, K.; Beckage, B.; Scheiner, S.; Molofsky, J. The ubiquity of phenotypic plasticity in plants: A synthesis. Ecol. Evol. 2015, 5, 3389–3400.
- van Kleunen, M.; Fischer, M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2005, 166, 49–60.
- Wilson, R.S.; Franklin, C.E. The detrimental acclimation hypothesis—Response. Trends Ecol. Evol. 2002, 17, 408.
- Acasuso-Rivero, C.; Murren, C.J.; Schlichting, C.D.; Steiner, U.K. Adaptive phenotypic plasticity for life-history and less fitness-related traits. Proc. Biol. Sci. 2019, 286, 20190653.
- Loeschcke, V.; Hoffmann, A.A. The detrimental acclimation hypothesis. Trends Ecol. Evol. 2002, 17, 407–408.
- Lalejini, A.; Ferguson, A.J.; Grant, N.A.; Ofria, C. Adaptive phenotypic plasticity stabilizes evolution in fluctuating environments. Front. Ecol. Evol. 2021, 9, 715381.
- Romero-Mujalli, D.; Rochow, M.; Kahl, S.; Paraskevopoulou, S.; Folkertsma, R.; Jeltsch, F.; Tiedemann, R. Adaptive and nonadaptive plasticity in changing environments: Implications for sexual species with different life history strategies. Ecol. Evol. 2021, 11, 6341–6357.
- Gabriel, W.; Lynch, M. The selective advantage of reaction norms for environmental tolerance. J. Evol. Biol. 1992, 5, 41–59.
- Scharloo, W. Developmental and physiological aspects of reaction norms—Drosophila data link genetic variation and phenotypic response to the environment. BioScience 1989, 39, 465–471.
- Schulte, P.M. What is environmental stress Insights from fish living in a variable environment. J. Exp. Biol. 2014, 217, 23–34.
- Ecker, S.; Pancaldi, V.; Valencia, A.; Beck, S.; Paul, D.S. Epigenetic and transcriptional variability shape phenotypic plasticity. Bioessays 2018, 40, 1700148.
- Conlon, I.; Raff, M. Size control in animal development. Cell 1999, 96, 235–244.
- Costanzo, J.P.; Lee, R.E. Avoidance and tolerance of freezing in ectothermic vertebrates. J. Exp. Biol. 2013, 216, 1961–1967.
- Duman, J.G. Animal ice-binding (antifreeze) proteins and glycolipids: An overview with emphasis on physiological function. J. Exp. Biol. 2015, 218, 1846–1855.
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257.
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011, 91, 1123–1159.
- Prosser, C.L. Adaptational Biology: Molecules to Organisms; Wiley: New York, NY, USA, 1986.
- Bradshaw, W.E.; Holzapfel, C.M. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 2010, 72, 147–166.
- Rosenthal, J.J.C. The emerging role of RNA editing in plasticity. J. Exp. Biol. 2015, 218, 1812–1821.
- Lamka, G.F.; Harder, A.M.; Sundaram, M.; Schwartz, T.S.; Christie, M.R.; DeWoody, J.A.; Willoughby, J.R. Epigenetics in ecology, evolution, and conservation. Front. Ecol. Evol. 2022, 10, 871791.
- Bryant, P.J.; Simpson, P. Intrinsic and extrinsic control of growth in developing organs. Q. Rev. Biol. 1984, 59, 387–415.
- Crickmore, M.A.; Mann, R.S. The control of size in animals: Insights from selector genes. Bioessays 2008, 30, 843–853.
- Hafen, E.; Stocker, H. How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 2003, 1, e86.
- Nijhout, H.F.; Riddiford, L.M.; Mirth, C.; Shingleton, A.W.; Suzuki, Y.; Callier, V. The developmental control of size in insects. Wiley Interdiscip. Rev. Dev. Biol. 2014, 3, 113–134.
- Parker, J. Morphogens, nutrients, and the basis of organ scaling. Evol. Dev. 2011, 13, 304–314.
- Potter, C.J.; Xu, T. Mechanisms of size control. Curr. Opin. Genet. Dev. 2001, 11, 279–286.
- Yang, X.; Xu, T. Molecular mechanism of size control in development and human diseases. Cell Res. 2011, 21, 715–729.
- Lessells, C.K. Neuroendocrine control of life histories: What do we need to know to understand the evolution of phenotypic plasticity? Philos. Trans. R. Soc. B 2008, 363, 1589–1598.
- Miettinen, T.P.; Caldez, M.J.; Kaldis, P.; Björklund, M. Cell size control—A mechanism for maintaining fitness and function. Bioessays 2017, 39, 1700058.
- Costantini, D.; Metcalfe, N.B.; Monaghan, P. Ecological processes in a hormetic framework. Ecol. Lett. 2010, 13, 1435–1447.
- Fischer, K.; Karl, I. Exploring plastic and genetic responses to temperature variation using copper butterflies. Clim. Res. 2010, 43, 17–30.
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81.
- Fischer, K.; Eenhoorn, E.; Bot, A.N.M.; Brakefield, P.M.; Zwaan, B.J. Cooler butterflies lay larger eggs: Developmental plasticity versus acclimation. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 2051–2056.
- Nijhout, H.F. Development and evolution of adaptive polyphenisms. Evol. Dev. 2003, 5, 9–18.
- Aubry, L.M.; Williams, C.T. Vertebrate phenological plasticity: From molecular mechanisms to ecological and evolutionary implications. Integr. Comp. Biol. 2022, 62, 958–971.
- Fu, R.; Gillen, A.E.; Grabek, K.R.; Riemondy, K.A.; Epperson, L.E.; Bustamante, C.D.; Hesselberth, J.R.; Martin, S.L. Dynamic RNA regulation in the brain underlies physiological plasticity in a hibernating mammal. Front. Physiol. 2021, 11, 624677.
- Piersma, T.; Drent, J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003, 18, 228–233.
- Piersma, T.; Lindström, A. Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol. Evol. 1997, 12, 134–138.
- Piersma, T.; van Gils, J.A. The Flexible Phenotype—A Body-Centered Integration of Ecology, Physiology, and Behaviour; Oxford University Press: Oxford, NY, USA, 2011.
- Hildebrandt, J.-P.; Wiesenthal, A.A.; Müller, C. Phenotypic plasticity in animals exposed to osmotic stress—Is it always adaptive? Bioessays 2018, 40, e1800069.




