
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Frankie Chi Fat Ko | -- | 2093 | 2023-06-19 03:21:14 | | | |
| 2 | Peter Tang | Meta information modification | 2093 | 2023-06-19 03:30:10 | | |
Video Upload Options
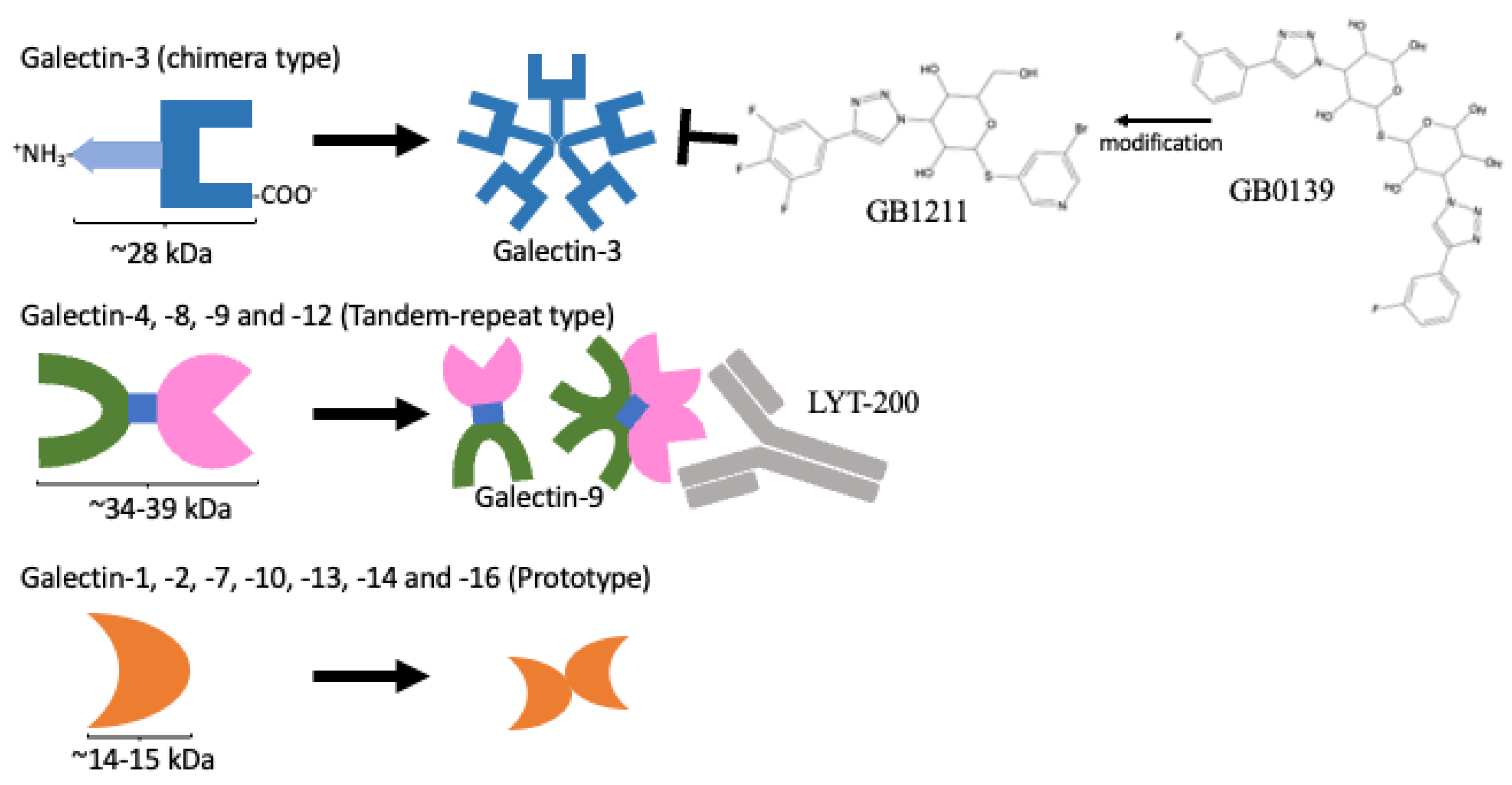
In humans, a total of 12 galectins have been identified. These galectins play important roles in controlling immune responses within the tumour microenvironment (TME) and the infiltration of immune cells, including different subsets of T cells, macrophages, and neutrophils, to fight against cancer cells. However, these infiltrating cells also have repair roles and are hijacked by cancer cells for pro-tumorigenic activities. Upon a better understanding of the immunomodulating functions of galectin-3 and -9, their inhibitors, namely, GB1211 and LYT-200, have been selected as candidates for clinical trials. The use of these galectin inhibitors as combined treatments with current immune checkpoint inhibitors (ICIs) is also undergoing clinical trial investigations. Through their network of binding partners, inhibition of galectin have broad downstream effects acting on CD8+ cytotoxic T cells, regulatory T cells (Tregs), Natural Killer (NK) cells, and macrophages as well as playing pro-inflammatory roles, inhibiting T-cell exhaustion to support the fight against cancer cells.
1. Introduction
|
Target |
Drug |
Phase |
Cancer Type |
Intervention |
|---|---|---|---|---|
|
Galectin-1 |
OTX008 |
I |
Solid tumours |
NCT01724320 Status unknown (updated: 2012) |
|
Galectin-3 |
Belapectin (GR-MD-02) |
I |
Metastatic melanoma |
NCT02117362 Completed (updated: 2019) |
|
I |
Metastatic melanoma, NSCLC, HNSCC |
NCT02575404 [21] Active (updated: 2022) |
||
|
GB1211 |
I |
Healthy subjects |
NCT03809052 [16] Completed (updated: 2021) |
|
|
I/II |
NSCLC |
NCT05240131 Recruiting (updated: 2023) |
||
|
GCS-100 |
I/II |
Relapsed/Refractory diffuse large-B-cell lymphoma |
NCT00776802 Withdrawn as funding issue (updated: 2013) |
|
|
GM-CT-01 |
I |
Breast, colorectal, head and neck, lung, prostate |
NCT00054977 Completed (updated: 2012) |
|
|
PectaSol-C, modified citrus pectin (MCP) [22] |
N/A |
Non-cancer-related: study for high blood pressure control |
NCT01960946 Completed (updated: 2021) |
|
|
Galactomannan/ ProLectin-M [23] |
III |
Non-cancer-related: antagonist for COVID-19 |
NCT05096052 Recruiting (updated: 2022) |
|
|
Galectin-9 |
LYT-200 (monoclonal antibody against galectin-9) |
I |
Acute myeloid leukaemia |
NCT05829226 Recruiting (updated: 2023) |
|
I/II |
Metastatic cancer in head and neck, colorectal, pancreatic, or urothelial origins |
NCT04666688 Recruiting (updated: 2023) |
2. Human Galectins
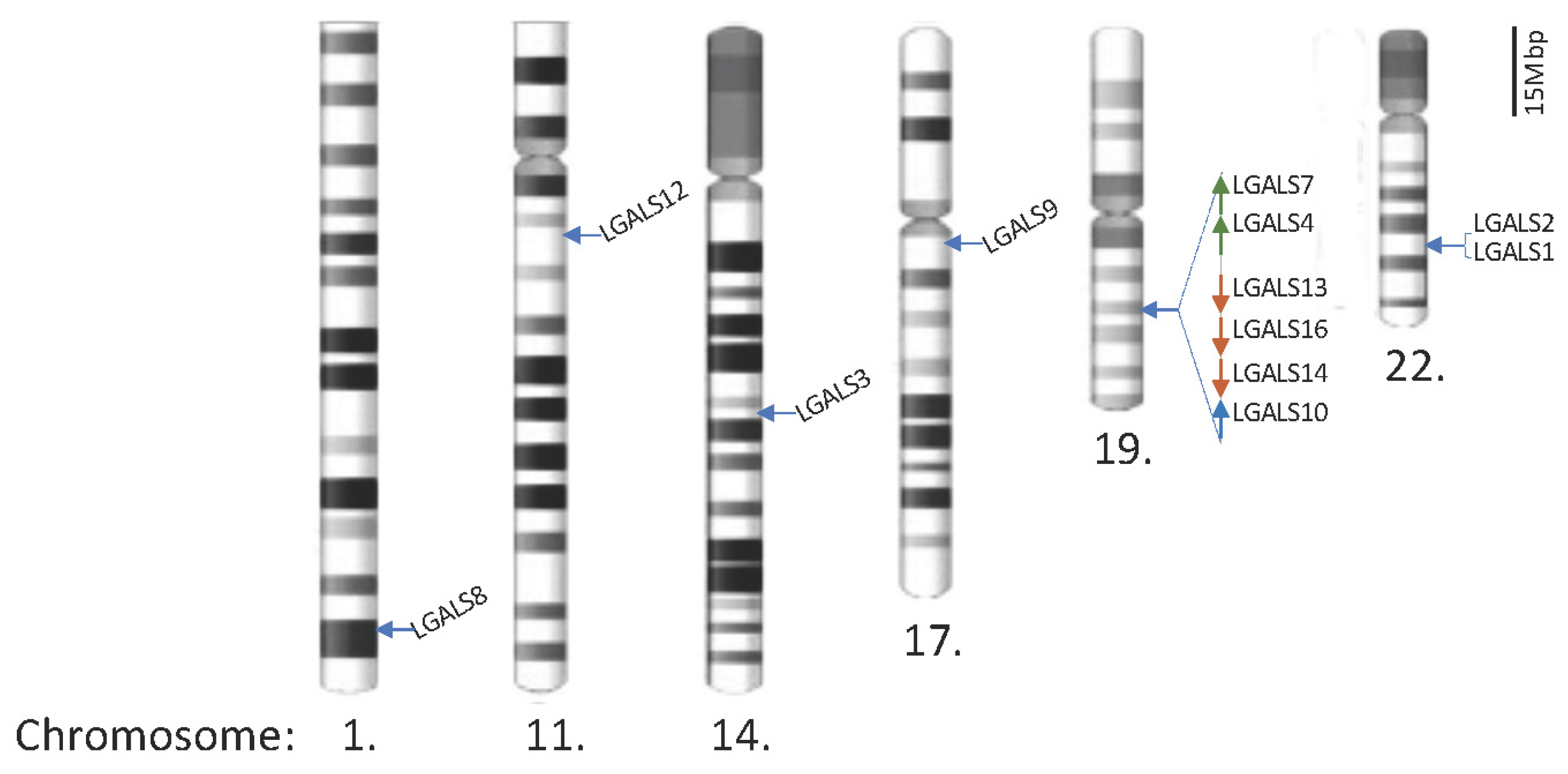
|
Gene/Protein Name (Chromosome Position [30]) |
Intracellular (Cytoplasmic/Nucleus) |
Extracellular |
|---|---|---|
|
LGALS1/Galectin-1 (Chr. 22q13.1) |
Cytoplasmic: GRP78 [31] Gemin4 [32] H-Ras [33] PCDH24 [34] |
CC and CXC chemokines [35] NRP1 [39] VEGFR2 [40] |
|
LGALS2/Galectin-2 (Chr. 22q13.1) |
Binds to surface of CD14(interm.–high) monocyte and promote M1 macrophage differentiation [41] |
|
|
LGALS3/Galectin-3 (Chr. 14q22.3) |
Cytoplasmic: Alix (EGFR trafficking) [42][43][44] Gemin4 [32] PCDH24 [34] Nucleus: hnRNPA2B1 [47] Sp1 [48] |
CC and CXC chemokines [35] CD29 [49] CD43 [49] CD45 [49] CD71 [49] EGFR [50] Interferon-γ [51] Integrin αvβ3 [46] LAG3 [52] MUC1 [53] |
|
LGALS4/Galectin-4 (Chr. 19q13.2) |
CD3 [54] |
|
|
LGALS7/Galectin-7 (Chr. 19q13.2) |
Cytoplasmic: Bcl-2 [55] |
|
|
LGALS8/Galectin-8 (Chr. 1q43) |
αM (CD11b, neutrophils) [56] CD166 [57] |
|
|
LGALS9/Galectin-9 (Chr. 17q11.2) |
Cytoplasmic: Binding to intracellular TIM-3 to modulate mTOR phosphorylation [60] Cytoplasmic–Lysosomes: Interact with Lamp2 to regulate lysosomal functions and autophagy [61] |
4-1BB [62] CD40 [63] CD44 [64] CD206 [65] Dectin-1 (macrophages) [66] DR3 [67] PD-1 [68] VISTA [75] |
|
LGALS10/Galectin-10/Charcot-Leyden crystal protein CLC (Chr. 19q13.2) |
Cytoplasmic–Granules: Eosinophil-derived neurotoxin EDN (RNS2) and eosinophil cationic protein ECP (RNS3) co-localised with CD63. It is required for the maturation of eosinophil during granulogenesis [76] |
|
|
LGALS12/Galectin-12/GRIP1 (Chr. 11q12.3) |
Cytoplasmic–Endosome/Lysosomes: VPS13C in lipid droplets and promotes the polarisation to M1 macrophage via TLR4 pathway [77][78] |
|
|
LGALS13/Galectin-13/ placental protein 13 (Chr. 19q13.2) |
Nucleus: HOXA1 [79] |
Binds to T lymphocytes and induces apoptosis [80]; Binds to neutrophils and shifts to immunoregulatory phenotype and promotes high PD-L1 expression [81] |
|
LGALS14/Galectin-14 (Chr. 19q13.2) |
Binds to T lymphocytes and induces apoptosis [80] c-Rel [82] |
|
|
LGALS16/Galectin-16 (Chr. 19q13.2) |
c-Rel [83] |
Remarks: Colour code is based on the galectin’s structure: chimera type (galectin-3) is highlighted in light blue; tandem-repeat type is highlighted in light green; prototype has not been highlighted.
3. From Bench to Bedside
3.1. Availability of Galectin-Specific Inhibitors
3.2. Applications, Safety/Pitfalls/Limitations, and Ongoing Clinical Trials
References
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545.
- Coley, W.B. II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199–220.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39.
- Pandey, P.; Khan, F.; Qari, H.A.; Upadhyay, T.K.; Alkhateeb, A.F.; Oves, M. Revolutionization in Cancer Therapeutics via Targeting Major Immune Checkpoints PD-1, PD-L1 and CTLA-4. Pharmaceuticals 2022, 15, 335.
- Chang, E.; Pelosof, L.; Lemery, S.; Gong, Y.; Goldberg, K.B.; Farrell, A.T.; Keegan, P.; Veeraraghavan, J.; Wei, G.; Blumenthal, G.M.; et al. Systematic Review of PD-1/PD-L1 Inhibitors in Oncology: From Personalized Medicine to Public Health. Oncologist 2021, 26, e1786–e1799.
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790.
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371.
- Preillon, J.; Cuende, J.; Rabolli, V.; Garnero, L.; Mercier, M.; Wald, N.; Pappalardo, A.; Denies, S.; Jamart, D.; Michaux, A.C.; et al. Restoration of T-cell Effector Function, Depletion of Tregs, and Direct Killing of Tumor Cells: The Multiple Mechanisms of Action of a-TIGIT Antagonist Antibodies. Mol. Cancer Ther. 2021, 20, 121–131.
- Florou, V.; Garrido-Laguna, I. Clinical Development of Anti-TIGIT Antibodies for Immunotherapy of Cancer. Curr. Oncol. Rep. 2022, 24, 1107–1112.
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792.
- Brazel, D.; Ou, S.I.; Nagasaka, M. Tiragolumab (Anti-TIGIT) in SCLC: Skyscraper-02, a Towering Inferno. Lung Cancer Targets Ther. 2023, 14, 1–9.
- Marino, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316.
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J. Med. Chem. 2022, 65, 12626–12638.
- Aslanis, V.; Slack, R.J.; MacKinnon, A.C.; McClinton, C.; Tantawi, S.; Gravelle, L.; Nilsson, U.J.; Leffler, H.; Brooks, A.; Khindri, S.K.; et al. Safety and pharmacokinetics of GB1211, an oral galectin-3 inhibitor: A single- and multiple-dose first-in-human study in healthy participants. Cancer Chemother. Pharmacol. 2023, 91, 267–280.
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477.
- Greer, P.F.C.; Rich, A.; Coates, D.E. Effects of galectin-1 inhibitor OTX008 on oral squamous cell carcinoma cells in vitro and the role of AP-1 and the MAPK/ERK pathway. Arch. Oral. Biol. 2022, 134, 105335.
- Koonce, N.A.; Griffin, R.J.; Dings, R.P.M. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. Int. J. Mol. Sci. 2017, 18, 2671.
- Leung, Z.; Ko, F.C.F.; Tey, S.K.; Kwong, E.M.L.; Mao, X.; Liu, B.H.M.; Ma, A.P.Y.; Fung, Y.M.E.; Che, C.M.; Wong, D.K.H.; et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. 2019, 38, 423.
- Curti, B.D.; Koguchi, Y.; Leidner, R.S.; Rolig, A.S.; Sturgill, E.R.; Sun, Z.; Wu, Y.; Rajamanickam, V.; Bernard, B.; Hilgart-Martiszus, I.; et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J. Immunother. Cancer 2021, 9, e002371.
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; Lopez, B.; Diez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21.
- Sigamani, A.; Mayo, K.H.; Miller, M.C.; Chen-Walden, H.; Reddy, S.; Platt, D. An Oral Galectin Inhibitor in COVID-19-A Phase II Randomized Controlled Trial. Vaccines 2023, 11, 731.
- von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 2006, 7, 909–918.
- Nielsen, H.; Tsirigos, K.D.; Brunak, S.; von Heijne, G. A Brief History of Protein Sorting Prediction. Protein J. 2019, 38, 200–216.
- Zanetti, G.; Pahuja, K.B.; Studer, S.; Shim, S.; Schekman, R. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 2011, 14, 20–28.
- Popa, S.J.; Stewart, S.E.; Moreau, K. Unconventional secretion of annexins and galectins. Semin. Cell Dev. Biol. 2018, 83, 42–50.
- Kutzner, T.J.; Higuero, A.M.; Sussmair, M.; Kopitz, J.; Hingar, M.; Diez-Revuelta, N.; Caballero, G.G.; Kaltner, H.; Lindner, I.; Abad-Rodriguez, J.; et al. How presence of a signal peptide affects human galectins-1 and -4: Clues to explain common absence of a leader sequence among adhesion/growth-regulatory galectins. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129449.
- Wan, L.; Hsu, Y.A.; Wei, C.C.; Liu, F.T. Galectins in allergic inflammatory diseases. Mol. Aspects Med. 2021, 79, 100925.
- Ensembl—Galectins. Available online: https://www.ensembl.org/Homo_sapiens/Search/Results?q=galectins;site=ensembl;facet_species=Human (accessed on 2 April 2023).
- Zhang, Q.; Ali, M.; Wang, Y.; Sun, Q.N.; Zhu, X.D.; Tang, D.; Wang, W.; Zhang, C.Y.; Zhou, H.H.; Wang, D.R. Galectin-1 binds GRP78 to promote the proliferation and metastasis of gastric cancer. Int. J. Oncol. 2022, 61, 1–18.
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 29, 3595–3602.
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493.
- Ose, R.; Oharaa, O.; Nagase, T. Galectin-1 and Galectin-3 Mediate Protocadherin-24-Dependent Membrane Localization of beta-catenin in Colon Cancer Cell Line HCT116. Curr. Chem. Genom. 2012, 6, 18–26.
- Eckardt, V.; Miller, M.C.; Blanchet, X.; Duan, R.; Leberzammer, J.; Duchene, J.; Soehnlein, O.; Megens, R.T.; Ludwig, A.K.; Dregni, A.; et al. Chemokines and galectins form heterodimers to modulate inflammation. EMBO Rep. 2020, 21, e47852.
- Fulcher, J.A.; Chang, M.H.; Wang, S.; Almazan, T.; Hashimi, S.T.; Eriksson, A.U.; Wen, X.; Pang, M.; Baum, L.G.; Singh, R.R.; et al. Galectin-1 co-clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase C signaling. J. Biol. Chem. 2009, 284, 26860–26870.
- Hernandez, J.D.; Nguyen, J.T.; He, J.; Wang, W.; Ardman, B.; Green, J.M.; Fukuda, M.; Baum, L.G. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J. Immunol. 2006, 177, 5328–5336.
- Auvynet, C.; Moreno, S.; Melchy, E.; Coronado-Martinez, I.; Montiel, J.L.; Aguilar-Delfin, I.; Rosenstein, Y. Galectin-1 promotes human neutrophil migration. Glycobiology 2013, 23, 32–42.
- Hsieh, S.H.; Ying, N.W.; Wu, M.H.; Chiang, W.F.; Hsu, C.L.; Wong, T.Y.; Jin, Y.T.; Hong, T.M.; Chen, Y.L. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008, 27, 3746–3753.
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Mendez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; Garcia-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014, 156, 744–758.
- Yildirim, C.; Vogel, D.Y.; Hollander, M.R.; Baggen, J.M.; Fontijn, R.D.; Nieuwenhuis, S.; Haverkamp, A.; de Vries, M.R.; Quax, P.H.; Garcia-Vallejo, J.J.; et al. Galectin-2 induces a proinflammatory, anti-arteriogenic phenotype in monocytes and macrophages. PLoS ONE 2015, 10, e0124347.
- Liu, W.; Hsu, D.K.; Chen, H.Y.; Yang, R.Y.; Carraway, K.L., 3rd; Isseroff, R.R.; Liu, F.T. Galectin-3 regulates intracellular trafficking of EGFR through Alix and promotes keratinocyte migration. J. Investig. Dermatol. 2012, 132, 2828–2837.
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035.
- Chen, H.Y.; Fermin, A.; Vardhana, S.; Weng, I.C.; Lo, K.F.; Chang, E.Y.; Maverakis, E.; Yang, R.Y.; Hsu, D.K.; Dustin, M.L.; et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 14496–14501.
- Mysore, V.P.; Zhou, Z.W.; Ambrogio, C.; Li, L.; Kapp, J.N.; Lu, C.; Wang, Q.; Tucker, M.R.; Okoro, J.J.; Nagy-Davidescu, G.; et al. A structural model of a Ras-Raf signalosome. Nat. Struct. Mol. Biol. 2021, 28, 847–857.
- Seguin, L.; Camargo, M.F.; Wettersten, H.I.; Kato, S.; Desgrosellier, J.S.; von Schalscha, T.; Elliott, K.C.; Cosset, E.; Lesperance, J.; Weis, S.M.; et al. Galectin-3, a Druggable Vulnerability for KRAS-Addicted Cancers. Cancer Discov. 2017, 7, 1464–1479.
- Fritsch, K.; Mernberger, M.; Nist, A.; Stiewe, T.; Brehm, A.; Jacob, R. Galectin-3 interacts with components of the nuclear ribonucleoprotein complex. BMC Cancer 2016, 16, 502.
- Jia, W.; Kong, L.; Kidoya, H.; Naito, H.; Muramatsu, F.; Hayashi, Y.; Hsieh, H.Y.; Yamakawa, D.; Hsu, D.K.; Liu, F.T.; et al. Indispensable role of Galectin-3 in promoting quiescence of hematopoietic stem cells. Nat. Commun. 2021, 12, 2118.
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789.
- Kuo, H.Y.; Hsu, H.T.; Chen, Y.C.; Chang, Y.W.; Liu, F.T.; Wu, C.W. Galectin-3 modulates the EGFR signalling-mediated regulation of Sox2 expression via c-Myc in lung cancer. Glycobiology 2016, 26, 155–165.
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793.
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423.
- Piyush, T.; Chacko, A.R.; Sindrewicz, P.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017, 24, 1937–1947.
- Paclik, D.; Danese, S.; Berndt, U.; Wiedenmann, B.; Dignass, A.; Sturm, A. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE 2008, 3, e2629.
- Villeneuve, C.; Baricault, L.; Canelle, L.; Barboule, N.; Racca, C.; Monsarrat, B.; Magnaldo, T.; Larminat, F. Mitochondrial proteomic approach reveals galectin-7 as a novel BCL-2 binding protein in human cells. Mol. Biol. Cell 2011, 22, 999–1013.
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin alphaM. Glycobiology 2003, 13, 755–763.
- Renard, H.F.; Tyckaert, F.; Lo Giudice, C.; Hirsch, T.; Valades-Cruz, C.A.; Lemaigre, C.; Shafaq-Zadah, M.; Wunder, C.; Wattiez, R.; Johannes, L.; et al. Endophilin-A3 and Galectin-8 control the clathrin-independent endocytosis of CD166. Nat. Commun. 2020, 11, 1457.
- Bieniasz-Krzywiec, P.; Martin-Perez, R.; Ehling, M.; Garcia-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell. Metab. 2019, 30, 917–936.e910.
- Cueni, L.N.; Detmar, M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp. Cell Res. 2009, 315, 1715–1723.
- Goncalves Silva, I.; Ruegg, L.; Gibbs, B.F.; Bardelli, M.; Fruehwirth, A.; Varani, L.; Berger, S.M.; Fasler-Kan, E.; Sumbayev, V.V. The immune receptor Tim-3 acts as a trafficker in a Tim-3/galectin-9 autocrine loop in human myeloid leukemia cells. Oncoimmunology 2016, 5, e1195535.
- Sudhakar, J.N.; Lu, H.H.; Chiang, H.Y.; Suen, C.S.; Hwang, M.J.; Wu, S.Y.; Shen, C.N.; Chang, Y.M.; Li, F.A.; Liu, F.T.; et al. Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat. Commun. 2020, 11, 4286.
- Madireddi, S.; Eun, S.Y.; Lee, S.W.; Nemcovicova, I.; Mehta, A.K.; Zajonc, D.M.; Nishi, N.; Niki, T.; Hirashima, M.; Croft, M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J. Exp. Med. 2014, 211, 1433–1448.
- Vaitaitis, G.M.; Wagner, D.H., Jr. Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity. PLoS ONE 2012, 7, e38708.
- Wu, C.; Thalhamer, T.; Franca, R.F.; Xiao, S.; Wang, C.; Hotta, C.; Zhu, C.; Hirashima, M.; Anderson, A.C.; Kuchroo, V.K. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014, 41, 270–282.
- Enninga, E.A.L.; Chatzopoulos, K.; Butterfield, J.T.; Sutor, S.L.; Leontovich, A.A.; Nevala, W.K.; Flotte, T.J.; Markovic, S.N. CD206-positive myeloid cells bind galectin-9 and promote a tumor-supportive microenvironment. J. Pathol. 2018, 245, 468–477.
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.S.B.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567.
- Madireddi, S.; Eun, S.Y.; Mehta, A.K.; Birta, A.; Zajonc, D.M.; Niki, T.; Hirashima, M.; Podack, E.R.; Schreiber, T.H.; Croft, M. Regulatory T Cell-Mediated Suppression of Inflammation Induced by DR3 Signaling Is Dependent on Galectin-9. J. Immunol. 2017, 199, 2721–2728.
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Yao, J.; Li, H.; Yan, M.; Chang, W.C.; Hsu, J.M.; Cha, J.H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832.
- Schaefer, K.; Webb, N.E.; Pang, M.; Hernandez-Davies, J.E.; Lee, K.P.; Gonzalez, P.; Douglass, M.V.; Lee, B.; Baum, L.G. Galectin-9 binds to O-glycans on protein disulfide isomerase. Glycobiology 2017, 27, 878–887.
- Bi, S.; Hong, P.W.; Lee, B.; Baum, L.G. Galectin-9 binding to cell surface protein disulfide isomerase regulates the redox environment to enhance T-cell migration and HIV entry. Proc. Natl. Acad. Sci. USA 2011, 108, 10650–10655.
- Yang, R.; Sun, L.; Li, C.F.; Wang, Y.H.; Xia, W.; Liu, B.; Chu, Y.Y.; Bover, L.; Vien, L.; Hung, M.C. Development and characterization of anti-galectin-9 antibodies that protect T cells from galectin-9-induced cell death. J. Biol. Chem. 2022, 298, 101821.
- Colomb, F.; Giron, L.B.; Premeaux, T.A.; Mitchell, B.I.; Niki, T.; Papasavvas, E.; Montaner, L.J.; Ndhlovu, L.C.; Abdel-Mohsen, M. Galectin-9 Mediates HIV Transcription by Inducing TCR-Dependent ERK Signaling. Front. Immunol. 2019, 10, 267.
- Pang, N.; Alimu, X.; Chen, R.; Muhashi, M.; Ma, J.; Chen, G.; Zhao, F.; Wang, L.; Qu, J.; Ding, J. Activated Galectin-9/Tim3 promotes Treg and suppresses Th1 effector function in chronic lymphocytic leukemia. FASEB J. 2021, 35, e21556.
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252.
- Yasinska, I.M.; Meyer, N.H.; Schlichtner, S.; Hussain, R.; Siligardi, G.; Casely-Hayford, M.; Fiedler, W.; Wellbrock, J.; Desmet, C.; Calzolai, L.; et al. Ligand-Receptor Interactions of Galectin-9 and VISTA Suppress Human T Lymphocyte Cytotoxic Activity. Front. Immunol. 2020, 11, 580557.
- Grozdanovic, M.M.; Doyle, C.B.; Liu, L.; Maybruck, B.T.; Kwatia, M.A.; Thiyagarajan, N.; Acharya, K.R.; Ackerman, S.J. Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis. J. Allergy Clin. Immunol. 2020, 146, 377–389.e10.
- Wan, L.; Lin, H.J.; Huang, C.C.; Chen, Y.C.; Hsu, Y.A.; Lin, C.H.; Lin, H.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744.
- Hancock-Cerutti, W.; Wu, Z.; Xu, P.; Yadavalli, N.; Leonzino, M.; Tharkeshwar, A.K.; Ferguson, S.M.; Shadel, G.S.; De Camilli, P. ER-lysosome lipid transfer protein VPS13C/PARK23 prevents aberrant mtDNA-dependent STING signaling. J. Cell Biol. 2022, 221, e202106046.
- Yang, T.; Yao, Y.; Wang, X.; Li, Y.; Si, Y.; Li, X.; Ayala, G.J.; Wang, Y.; Mayo, K.H.; Tai, G.; et al. Galectin-13/placental protein 13: Redox-active disulfides as switches for regulating structure, function and cellular distribution. Glycobiology 2020, 30, 120–129.
- Balogh, A.; Toth, E.; Romero, R.; Parej, K.; Csala, D.; Szenasi, N.L.; Hajdu, I.; Juhasz, K.; Kovacs, A.F.; Meiri, H.; et al. Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response. Front. Immunol. 2019, 10, 1240.
- Vokalova, L.; Balogh, A.; Toth, E.; Van Breda, S.V.; Schafer, G.; Hoesli, I.; Lapaire, O.; Hahn, S.; Than, N.G.; Rossi, S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020, 11, 145.
- Si, Y.; Li, Y.; Yang, T.; Li, X.; Ayala, G.J.; Mayo, K.H.; Tai, G.; Su, J.; Zhou, Y. Structure-function studies of galectin-14, an important effector molecule in embryology. FEBS J. 2021, 288, 1041–1055.
- Si, Y.; Yao, Y.; Jaramillo Ayala, G.; Li, X.; Han, Q.; Zhang, W.; Xu, X.; Tai, G.; Mayo, K.H.; Zhou, Y.; et al. Human galectin-16 has a pseudo ligand binding site and plays a role in regulating c-Rel-mediated lymphocyte activity. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129755.
- Miller, M.C.; Nesmelova, I.V.; Daragan, V.A.; Ippel, H.; Michalak, M.; Dregni, A.; Kaltner, H.; Kopitz, J.; Gabius, H.J.; Mayo, K.H. Pro4 prolyl peptide bond isomerization in human galectin-7 modulates the monomer-dimer equilibrum to affect function. Biochem. J. 2020, 477, 3147–3165.
- Laderach, D.J.; Compagno, D. Inhibition of galectins in cancer: Biological challenges for their clinical application. Front. Immunol. 2022, 13, 1104625.
- Bertuzzi, S.; Gimeno, A.; Martinez-Castillo, A.; Lete, M.G.; Delgado, S.; Airoldi, C.; Rodrigues Tavares, M.; Blahova, M.; Chytil, P.; Kren, V.; et al. Cross-Linking Effects Dictate the Preference of Galectins to Bind LacNAc-Decorated HPMA Copolymers. Int. J. Mol. Sci. 2021, 22, 6000.
- Wang, T.; Cai, S.; Cheng, Y.; Zhang, W.; Wang, M.; Sun, H.; Guo, B.; Li, Z.; Xiao, Y.; Jiang, S. Discovery of Small-Molecule Inhibitors of the PD-1/PD-L1 Axis That Promote PD-L1 Internalization and Degradation. J. Med. Chem. 2022, 65, 3879–3893.
- Croese, T.; Castellani, G.; Schwartz, M. Immune cell compartmentalization for brain surveillance and protection. Nat. Immunol. 2021, 22, 1083–1092.
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2.
- Eruslanov, E.B.; Singhal, S.; Albelda, S.M. Mouse versus Human Neutrophils in Cancer: A Major Knowledge Gap. Trends Cancer 2017, 3, 149–160.
- Douam, F.; Ploss, A. A humanized “new-trophil” mouse to study early inflammatory processes. Proc. Natl. Acad. Sci. USA 2022, 119, e2216699119.
- Diaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm. 2017, 2017, 9247574.
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Nishio, M.; Mok, T.S.K.; Reck, M.; Finley, G.G.; Kaul, M.D.; Yu, W.; Paranthaman, N.; et al. Association of Immune-Related Adverse Events With Efficacy of Atezolizumab in Patients With Non-Small Cell Lung Cancer: Pooled Analyses of the Phase 3 IMpower130, IMpower132, and IMpower150 Randomized Clinical Trials. JAMA Oncol. 2023, 9, 527–535.




