
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Gryglewska-Wawrzak | -- | 1065 | 2023-06-17 12:06:44 | | | |
| 2 | Dean Liu | Meta information modification | 1065 | 2023-06-19 03:31:50 | | | | |
| 3 | Dean Liu | Meta information modification | 1065 | 2023-06-19 03:33:41 | | |
Video Upload Options
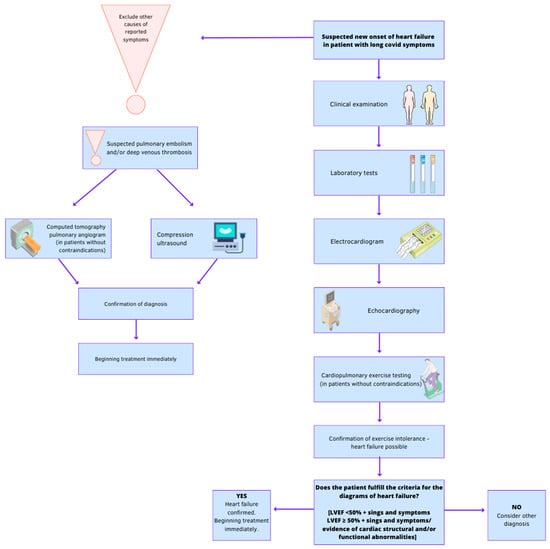
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It can lead to myocardial damage. Heart failure (HF) is a significant global health concern and is characterized as a clinical syndrome with symptoms caused by structural and/or functional abnormalities of the heart, confirmed by elevated natriuretic peptide levels and evidence of pulmonary or systemic congestion. The relationship between COVID-19 and heart failure is complex. SARS-CoV-2 can cause cardiac damage through the activation of pro-inflammatory cytokines. Understanding the interactions between the disease and viruses is crucial for optimal patient care. However, the validity of screening for cardiovascular complications after COVID-19 remains unconfirmed, and individualized diagnosis procedures are necessary based on the patient's clinical symptoms.
1. Clinical Examination
2. Laboratory Tests
-
concentration of natriuretic peptides in plasma—to exclude HF: in a patient without acute worsening of symptoms, HF is unlikely when BNP < 35 pg/mL (<105 pg/mL in atrial fibrillation), NT-proBNP < 125 pg/mL (<365 pg/mL in atrial fibrillation);
-
arterial blood gas analysis for detection of respiratory failure;
-
serum troponin for detection of acute coronary syndrome (ACS);
-
blood urea nitrogen, serum creatinine, electrolytes—for the detection of renal dysfunction;
-
full blood count—anemia may exacerbate or cause CHF;
-
transferrin, ferritin, signs of iron deficiency, most often of a functional nature—reduced transferrin iron saturation; a decrease in ferritin usually occurs only with absolute iron deficiency (it may not occur in the presence of inflammation);
-
inflammatory cytokines (C-reactive protein, procalcitonin)—for the diagnosis of infection;
-
increased activity of aminotransferases and lactate dehydrogenase (LDH), increased concentrations of bilirubin in plasma—in patients with venous stasis in the systemic circulation, with hepatomegaly;
-
the concentration of thyroid stimulating hormone (TSH), because thyroid disease can mimic or worsen the symptoms of HF;
-
D-dimer—when pulmonary embolism (PE) is suspected.
3. Electrocardiogram (ECG)
4. Chest Radiograph
5. Echocardiography
-
Left ventricular systolic function—by analysing segmental and global left ventricular contractility and left ventricular ejection fraction (LVEF) measurement (Simpson method; <40% indicates significant left ventricular systolic dysfunction; values 41–49% are considered the so-called grey zone and one of the diagnostic criteria HFmrEF—a complete differential diagnosis of noncardiac causes of symptoms is necessary, as in HFpEF) [3].
-
Left ventricular diastolic function—transmitral E/A ratio and E velocity deceleration time (DT), e’ velocity (average and absolute value of septal and lateral side) of the mitral annulus by pulsed tissue Doppler, E/e’ ratio, and the estimate of systolic pulmonary artery pressure (sPAP) derived from tricuspid regurgitation (TR) velocity [10].
-
Anatomical abnormalities, hypertrophy, dilation of the heart chambers, valvular defects, congenital defects. Additional evaluation of many parameters of cardiac structure and function is of particular importance in differential diagnosis, especially with LVEF <40%. In some cases (e.g., poor imaging conditions on transthoracic examination, suspected prosthetic valve dysfunction, detection of a thrombus in the left ear in patients with atrial fibrillation, diagnosis of bacterial endocarditis or congenital defects), transoesophageal echocardiography is indicated [11].
-
Signs of PE—dilation of the right ventricle (RV), pulmonary ejection acceleration time <60 ms with a peak systolic tricuspid valve gradient < 60 mmHg [4]. Echocardiographic examination is not mandatory as part of the routine diagnostic workup in haemodynamically stable patients with suspected PE. In case of suspected high-risk PE, the absence of echocardiographic signs of RV overload or dysfunction practically excludes PE as the cause of hemodynamic instability [12].
6. Computed Tomographic Pulmonary Angiography (CTPA)
7. Compression Ultrasonography (CUS)
8. Cardiopulmonary Exercise Testing (CPET)
References
- Harada, R.; Mantha, Y.; Hieda, M. Back to Basics: Key Physical Examinations and Theories in Patients with Heart Failure. Heart Fail. Clin. 2020, 16, 139–151.
- Thibodeau, J.T.; Drazner, M.H. The Role of the Clinical Examination in Patients with Heart Failure. JACC Heart Fail. 2018, 6, 543–551.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726.
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647.
- Carrier, M.; Righini, M.; Djurabi, R.K.; Huisman, M.V.; Perrier, A.; Wells, P.S.; Rodger, M.; Wuillemin, W.A.; Le Gal, G. VIDAS D-dimer in combination with clinical pre-test probability to rule out pulmonary embolism. A systematic review of management outcome studies. Thromb. Haemost. 2009, 101, 886–892.
- Righini, M.; Van Es, J.; Exter, P.D. Age-Adjusted D-Dimer Cutoff Levels to Rule Out Pulmonary Embolism: The ADJUST-PE Study. JAMA 2014, 311, 1117.
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: A systematic review and meta-analysis of 100 studies. Sci. Rep. 2021, 11, 21888.
- Gouda, P.; Brown, P.; Rowe, B.H.; McAlister, F.A.; Ezekowitz, J.A. Insights into the importance of the electrocardiogram in patients with acute heart failure. Eur. J. Heart Fail. 2016, 18, 1032–1040.
- Pan, D.; Pellicori, P.; Dobbs, K.; Bulemfu, J.; Sokoreli, I.; Urbinati, A.; Brown, O.; Sze, S.; Rigby, A.S.; Kazmi, S.; et al. Prognostic value of the chest X-ray in patients hospitalised for heart failure. Clin. Res. Cardiol. 2021, 110, 1743–1756.
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310.
- Szyszka, A.; Płońska-Gościniak, E. Transesophageal echocardiography. J. Ultrason. 2019, 19, 62–65.
- Roy, P.-M.; Colombet, I.; Durieux, P.; Chatellier, G.; Sors, H.; Meyer, G. Systematic review and meta-analysis of strategies for the diagnosis of suspected pulmonary embolism. BMJ 2005, 331, 259.
- Kearon, C. Natural History of Venous Thromboembolism. Circulation 2003, 107, I22–I30.
- Glaab, T.; Taube, C. Practical guide to cardiopulmonary exercise testing in adults. Respir. Res. 2022, 23, 9.
- Durstenfeld, M.S.; Sun, K.; Tahir, P.; Peluso, M.J.; Deeks, S.G.; Aras, M.A.; Grandis, D.J.; Long, C.S.; Beatty, A.; Hsue, P.Y. Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2236057.




