
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | giandomenico roviello | -- | 1702 | 2023-06-16 11:50:21 | | | |
| 2 | Jessie Wu | -1 word(s) | 1701 | 2023-06-19 05:21:12 | | |
Video Upload Options
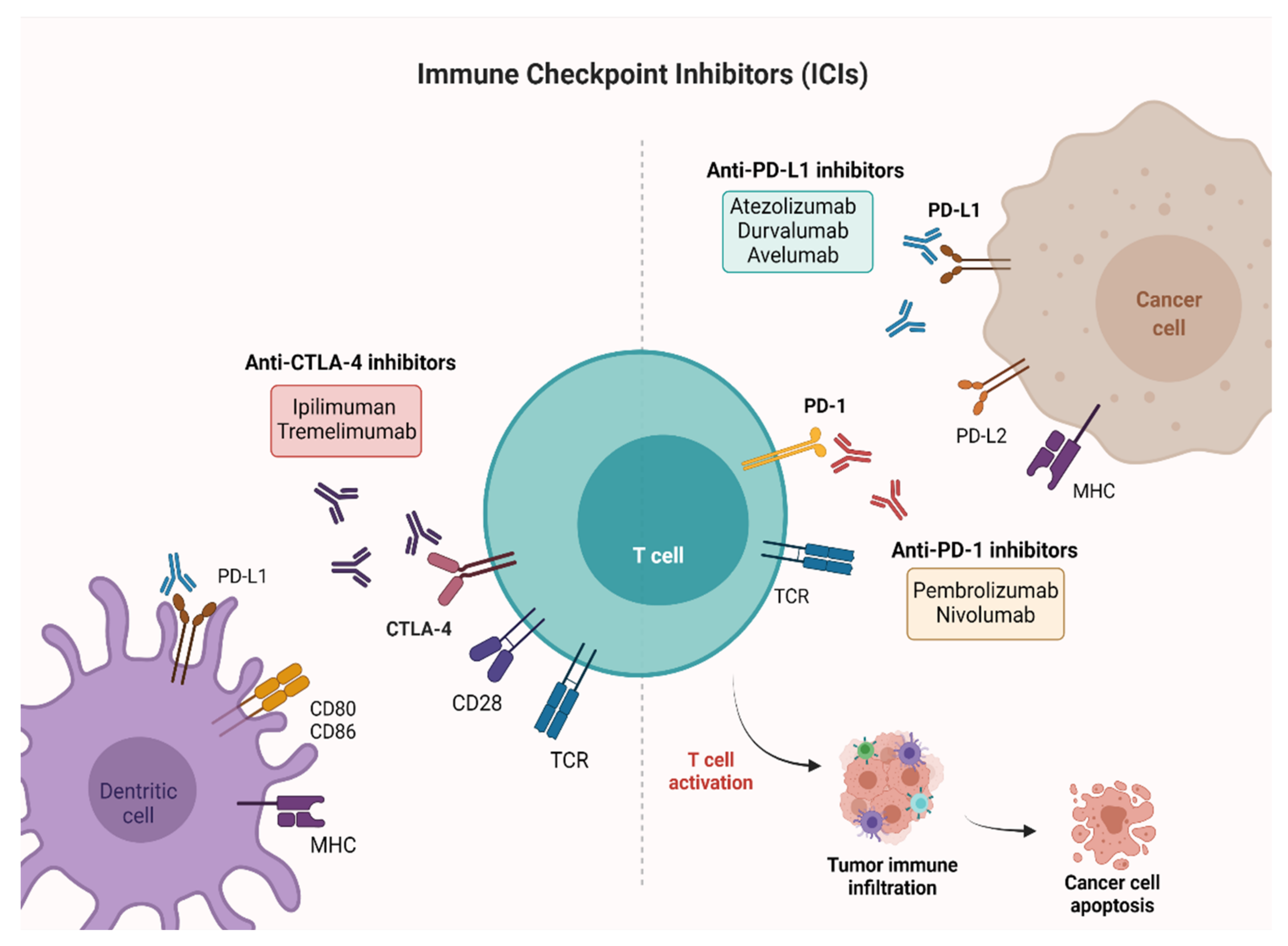
Bladder cancer (BC) is the most common malignancy of the genitourinary tract, with high morbidity and mortality rates. Until recently, the treatment of locally advanced or metastatic urothelial BC was based on the use of chemotherapy alone. Since 2016, five immune checkpoint inhibitors (ICIs) have been approved by the Food and Drug Administration (FDA) in different settings, i.e., first-line, maintenance and second-line treatment, while several trials are still ongoing in the perioperative context. Lately, pembrolizumab, a programmed death-1 (PD-1) inhibitor, has been approved for Bacillus Calmette–Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (NMIBC), using immunotherapy at an early stage of the disease.
1. Introduction
| Trial | Phase | FDA Approval | No. of Patients | ICI Therapy | Line of Treatment | Previous Platinum Therapy | Efficacy Outcomes |
|---|---|---|---|---|---|---|---|
| IMvigor210 [12] | II | May 2016 | 310 | Atezolizumab | Second line | Yes | mPFS: 2.1 mo mOS: 7.9 mo ORR: 18% |
| CheckMate-275 [13] | II | February 2017 | 265 | Nivolumab | Second line | Yes | mPFS: 2.0 mo mOS: 8.7 mo ORR: 20% |
| IMvigor210 [14] | II | April 2017 | 123 | Atezolizumab | First line PD-L1+ platinum ineligible patients | No | mPFS: 2.7 mo mOS: 15.9 mo ORR: 23% |
| JAVELIN Solid Tumor [15] | I | May 2017 | 44 | Avelumab | Second line | Yes | mPFS: 11.6 wk mOS: 13.7 mo ORR: 18.2% |
| Study 1108 [16] | I/II | May 2017 | 191 | Durvalumab | Second line | Yes | mPFS: 1.5 mo mOS: 18.2 mo ORR: 18% |
| KEYNOTE-045 [17] | III | May 2017 | 542 | Pembrolizumab | Second line | Yes | mPFS: 2.1 mo mOS: 10.3 mo ORR: 21% |
| KEYNOTE-052 [18] | II | May 2017 | 370 | Pembrolizumab | First line PD-L1 + platinum ineligible patients | No | mPFS: 2.2 mo mOS: 11.3 mo ORR: 29% |
| JAVELIN Bladder 100 [19] | III | June 2020 | 700 | Avelumab | Maintenance therapy | Yes | mPFS: 3.7 mo mOS: 21.4 mo |
2. Non-Muscle Invasive Bladder Cancer (NMIBC)
| Trial | Phase | Allocation | No. of Patients | Study Populations | Line of Treatment | Experimental Arms | Primary Outcome |
|---|---|---|---|---|---|---|---|
| NCT02736266 | II | N/A | 90 | MIBC | neoadjuvant prior to chemoradiation | Pembrolizumab | pCR |
| NCT02845323 | II | randomized | 44 | MIBC | neoadjuvant | Nivolumab + Urelumab vs. Nivolumab | Immune response (tumor infiltrating CD8+ T cell density) |
| NCT03520491 | II | not randomized | 45 | Cisplatin-ineligible patients with MIBC | neoadjuvant | Nivolumab and Nivolumab + Ipilimumab | No. of patients who proceed to RC-PLND |
| NCT03472274 | II | randomized | 99 | BC patients | neoadjuvant | Durvalumab and Tremelimumab | Antitumor activity |
| NCT03732677 | III | randomized | 1050 | MIBC | neoadjuvant/adjuvant | Durvalumab + Gemcitabine + Cisplatin neoadjuvant treatment followed by Durvalumab alone for adjuvant treatment | EFS |
| NCT04138628 | II | randomized | 282 | Treatment of mBC at the time of biochemical relapse following RC | adjuvant | Atezolizumab | CR |
| NCT03244384 | III | randomized | 739 | Locally advanced and mUC | adjuvant | Pembrolizumab vs. observation | OS, DFS |
| NCT04223856 | III | randomized | 760 | Previously untreated locally advanced or mUC | 1st | Enfortumab vedotin + Pembrolizumab vs. chemotherapy alone | PFS, OS |
| NCT03036098 | III | randomized | 1290 | Unresectable or mUC | 1st | Nivolumab + Ipilimumab, or SoC chemotherapy vs. SoC Chemotherapy | OS, PFS |
| NCT03682068 | III | randomized | 1434 | Unresectable locally advanced or mUC | 1st | Durvalumab + SoC chemotherapy and Durvalumab + Tremelimumab and SoC Chemotherapy vs. SoC chemotherapy alone | OS |
| NCT03898180 | III | randomized | 694 | Locally advanced or mUC | 1st | Pembrolizumab + Lenvatinib vs. Pembrolizumab +placebo | PFS, OS |
| NCT03697850 | II | randomized | 77 | MIBC patients ineligible for RC | maintenance therapy | Atezolizumab | DFS |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108.
- Cumberbatch, M.G.; Rota, M.; Catto, J.W.; La Vecchia, C. The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks. Eur. Urol. 2016, 70, 458–466.
- Polesel, J.; Bosetti, C.; di Maso, M.; Montella, M.; Libra, M.; Garbeglio, A.; Zucchetto, A.; Turati, F.; Talamini, R.; La Vecchia, C.; et al. Duration and intensity of tobacco smoking and the risk of papillary and non-papillary transitional cell carcinoma of the bladder. Cancer Causes Control 2014, 25, 1151–1158.
- Cumberbatch, M.G.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795.
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 Update. Eur. Urol. 2019, 76, 639–657.
- Ghandour, R.; Singla, N.; Lotan, Y. Treatment options and outcomes in nonmetastatic muscle invasive bladder cancer. Trends Cancer 2019, 5, 426–439.
- Kim, D.K.; Lee, J.Y.; Jung, J.H.; Hah, Y.S.; Cho, K.S. Role of adjuvant cisplatin-based chemotherapy following radical cystectomy in locally advanced muscle-invasive bladder cancer: Systematic review and meta-analysis of randomized trials. Investig. Clin. Urol. 2019, 60, 64–74.
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438.
- Von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 2000, 18, 3068–3077.
- Sternberg, C.N.; de Mulder, P.H.; Schornagel, J.H.; Théodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, F.; Spina, M.; van Groeningen, C.J.; de Balincourt, C.; et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J. Clin.Oncol. 2001, 19, 2638–2646.
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920.
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322.
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76.
- Apolo, A.B.; Infante, J.R.; Balmanoukian, A.; Patel, M.R.; Wang, D.; Kelly, K.; Mega, A.E.; Britten, C.D.; Ravaud, A.; Mita, A.C.; et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: Results from a multicenter, phase Ib study. J. Clin. Oncol. 2017, 35, 2117–2124.
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017, 3, e172411.
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026.
- Vuky, J.; Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-term outcomes in KEYNOTE-052: Phase II study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 2020, 38, 2658–2666.
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230.
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242.
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell Biol. 2005, 25, 9543–9553.
- Riella, L.V.; Paterson, A.M.; Sharpe, A.H.; Chandraker, A. Role of the PD-1 pathway in the immune response. Am. J. Transplant. 2012, 12, 2575–2587.
- Momtaz, P.; Postow, M.A. Immunologic checkpoints in cancer therapy: Focus on the programmed death-1 (PD-1) receptor pathway. Pharmgenom. Pers Med. 2014, 7, 357–365.
- Massari, F.; Santoni, M.; Ciccarese, C.; Santini, D.; Alfieri, S.; Martignoni, G.; Brunelli, M.; Piva, F.; Berardi, R.; Montironi, R.; et al. PD-1 blockade therapy in renal cell carcinoma: Current studies and future promises. Cancer Treat Rev. 2015, 41, 114–121.
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr. Opin. Immunol. 2012, 24, 207–212.
- Ribas, A. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 2012, 366, 2517–2519.
- Treatment by Cancer Type . . Available online: https://www.nccn.org/guidelines/category_1 (accessed on 1 July 2021).
- EAU Guidelines | Uroweb . . Available online: https://uroweb.org/guidelines (accessed on 1 July 2021).
- Balar, A.V.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.; Mourey, L.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Kamat, A.M.; Grivas, P.; et al. Keynote 057: Phase II trial of Pembrolizumab (pembro) for patients (pts) with high-risk (HR) nonmuscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guérin (BCG). J. Clin. Oncol. 2019, 37 (Suppl. S7), 350.
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Bajorin, D.F.; Roumiguié, M.; Singer, E.A.; Krieger, L.E.M.; Grivas, P.; et al. Pembrolizumab (pembro) for the treatment of patients with Bacillus Calmette-Guérin (BCG) unresponsive, high-risk (HR) non–muscle-invasive bladder cancer (NMIBC): Over two years follow-up of KEYNOTE-057. J. Clin. Oncol. 2020, 38 (Suppl. S15), 5041.
- Balar, A.V.; Kamat, A.M.; Kulkami, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab for the treatment of patients with high-risk (HR) non-muscle-invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guérin: Extended follow-up of KEYNOTE-057 cohort A. J. Clin. Oncol. 2021, 39 (Suppl. S6), 451.
- Black, P.C.; Tangen, C.; Singh, P.; McConkey, D.J.; Lucia, S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.; Sharon, E.; et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J. Clin. Oncol. 2020, 38 (Suppl. S15), 5022.
- Kamat, A.M.; Shore, N.; Hahn, N.; Alanee, S.; Nishiyama, H.; Shariat, S.; Nam, K.; Kapadia, E.; Frenkl, T.; Steinberg, G. KEYNOTE-676: Phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020, 16, 507–516.




