Bladder cancer (BC) is the most common malignancy of the genitourinary tract, with high morbidity and mortality rates. Until recently, the treatment of locally advanced or metastatic urothelial BC was based on the use of chemotherapy alone. Since 2016, five immune checkpoint inhibitors (ICIs) have been approved by the Food and Drug Administration (FDA) in different settings, i.e., first-line, maintenance and second-line treatment, while several trials are still ongoing in the perioperative context. Lately, pembrolizumab, a programmed death-1 (PD-1) inhibitor, has been approved for Bacillus Calmette–Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (NMIBC), using immunotherapy at an early stage of the disease.
1. Introduction
Bladder cancer (BC) is the ninth-most common malignancy worldwide, with 83,730 estimated new cases in the USA in 2021
[1] and the seventh-most common cancer in men
[2]. Tobacco smoke appears to be the most common risk factor for BC, accounting for approximately 50% of cases
[3]. Compared with never smokers, BC risk is three-fold higher in former smokers and over six-fold higher in current smokers, steadily increasing with the number of cigarettes and years smoked
[4]. Occupational exposure is responsible for 5–6% of urothelial carcinomas. Among dietary factors, alcohol appears to play a role in the pathogenesis of BC, while the intake of Vitamin D and daily consumption of fruit and vegetables could have a protective effect
[5].
At the time of diagnosis, approximately 70% of urothelial carcinomas are superficial, while 30% present with muscle infiltration
[2]. Treatment of non-muscle invasive bladder cancer (NMIBC) involves transurethral resection of the bladder tumor (TURBT) followed by intravesical chemotherapy or immunotherapy. Bacillus Calmette–Guérin (BCG) immunotherapy is the gold standard adjuvant treatment for NMIBC with a high risk of progression and is also recommended for intermediate-risk NMIBC
[6].
The standard treatment for nonmetastatic muscle invasive bladder cancer (MIBC) (T2–T4, N0, M0) is neoadjuvant cisplatin-based therapy, succeeded by radical cystectomy (RC) and pelvic lymphadenectomy
[7]. Patients undergoing RC for MIBC have a high risk of relapse, especially in cases of ≥pT2 disease and/or pathological lymph node involvement. Adjuvant cisplatin-based multi-chemotherapy may be considered for patients fulfilling platinum eligibility criteria that include at least one of the following: Eastern Cooperative Oncology Group (ECOG) performance status of 2, creatinine clearance less than 60 mL/min, grade ≥ 2 hearing loss, grade ≥ 2 neuropathy, and/or New York Heart Association Class III heart failure
[8][9][8,9].
Cisplatin-containing chemotherapy is the preferred first-line treatment also in metastatic disease. The most commonly used regimens in this setting include a combination of gemcitabine and cisplatin (GC), methotrexate, vincristine, adriamycin and cisplatin (MVAC) every four weeks, or dose-dense (dd) MVAC every two weeks. The median overall survival (OS) rates are 13.8 months, 14.8 months, and 15.5 months for GC, MVAC, and ddMVAC regimens, respectively
[10][11][10,11]. Outcome is poor for patients who are unfit for platinum chemotherapy or undergo progression after frontline platinum chemotherapy, however, a major milestone in the metastatic setting was the approval of immune checkpoint inhibitors (ICIs) (
Table 1).
Table 1.
Currently approved ICIs administered in urothelial bladder carcinoma.
| Trial |
Phase |
FDA Approval |
No. of Patients |
ICI Therapy |
Line of Treatment |
Previous Platinum Therapy |
Efficacy Outcomes |
| IMvigor210 [12] |
II |
May 2016 |
310 |
Atezolizumab |
Second line |
Yes |
mPFS: 2.1 mo
mOS: 7.9 mo
ORR: 18% |
| CheckMate-275 [13] |
II |
February 2017 |
265 |
Nivolumab |
Second line |
Yes |
mPFS: 2.0 mo
mOS: 8.7 mo
ORR: 20% |
| IMvigor210 [14] |
II |
April 2017 |
123 |
Atezolizumab |
First line PD-L1+ platinum ineligible patients |
No |
mPFS: 2.7 mo
mOS: 15.9 mo
ORR: 23% |
| JAVELIN Solid Tumor [15] |
I |
May 2017 |
44 |
Avelumab |
Second line |
Yes |
mPFS: 11.6 wk
mOS: 13.7 mo
ORR: 18.2% |
| Study 1108 [16] |
I/II |
May 2017 |
191 |
Durvalumab |
Second line |
Yes |
mPFS: 1.5 mo
mOS: 18.2 mo
ORR: 18% |
| KEYNOTE-045 [17] |
III |
May 2017 |
542 |
Pembrolizumab |
Second line |
Yes |
mPFS: 2.1 mo
mOS: 10.3 mo
ORR: 21% |
| KEYNOTE-052 [18] |
II |
May 2017 |
370 |
Pembrolizumab |
First line PD-L1 + platinum ineligible patients |
No |
mPFS: 2.2 mo
mOS: 11.3 mo
ORR: 29% |
| JAVELIN Bladder 100 [19] |
III |
June 2020 |
700 |
Avelumab |
Maintenance therapy |
Yes |
mPFS: 3.7 mo
mOS: 21.4 mo |
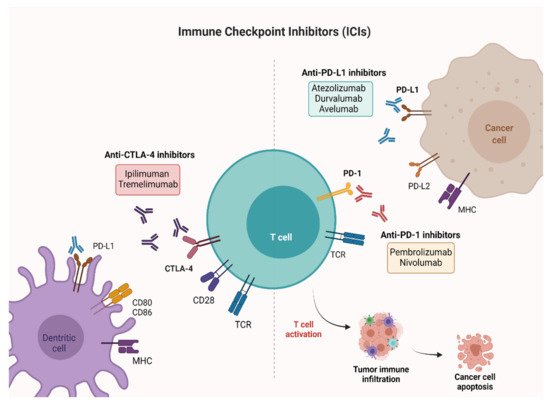
ICIs are monoclonal antibodies directed against cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), programmed death 1 (PD-1) receptor and programmed death ligand-1 (PD-L1). CTLA-4 is a membrane receptor acting as a major negative regulator of T cell responses through interaction with its ligands, CD80 (B7-1) and CD86 (B7-2), expressed on the surface of antigen-presenting cells. PD-1 is a membrane receptor expressed by T cells, particularly in conditions of chronic antigen exposure, and exerts an inhibitory action on lymphocytes by binding to its two ligands, PD-L1 and PD-L2. PD-L1 is expressed on immune cells, such as T cells, B cells, dendritic cells (DCs) and macrophages
[20][21][20,21], while PD-L2 is expressed mainly on antigen-presenting cells (APCs), including macrophages and myeloid DCs
[22][23][22,23]. PD-L1 and PD-L2 have differential functions in immune regulatory processes. Indeed, PD-L1 inhibits T cells in peripheral tissues, whereas PD-L2 suppresses immune T cell activation in lymphoid organs. PD-L2 also inhibits type 2 T-helper (T
H2) lymphocytes, but its role is yet to be fully understood
[24][25][24,25]. By interrupting the ligand/receptor interactions, the anti-CTLA-4 (ipilimumab, tremelimumab) and anti-PD-1 (nivolumab, pembrolizumab)/anti-PD-L1 (atezolizumab, durvalumab, avelumab) antibodies remove T cell inhibition, thus favoring antitumor cytotoxic activity
[26] (
Figure 1). Characterization of immune checkpoints has furthered development of novel immunotherapeutic agents with clinical activity against a variety of solid tumors, including BC.
Figure 1. Mechanisms of action of ICIs targeting PD-1, PD-L1, and CTLA-4. PD-1 and CTLA-4 are proteins expressed on activated T cells. Their binding to the respective ligands presented on the surface of cancer cells leads to T cell inactivation and prevents tumor cell death. The immune checkpoint blockade ensures the activation of T cells and favors antitumor activity. Created with
BioRender.com (accessed on 26 July 2021). PD-1: Programmed cell death-1; PD-L1: Programmed cell death-ligand 1; CTLA-4: Cytotoxic T-lymphocyte-associated antigen 4.
This aresearchticle reviews current evidence supporting the use of new checkpoint inhibitors in BC, along with information on biomarkers that may predict response to immunotherapy.
2. Non-Muscle Invasive Bladder Cancer (NMIBC)
In approximately 75% of BC patients, the disease is confined to the mucosa (stage Ta, carcinoma in situ) or submucosa (stage T1)
[27]. Although TURB alone can eradicate TaT1 tumors completely, they commonly recur and can progress to MIBC, thus necessitating the use of adjuvant treatment. In patients with intermediate-risk tumors, one-year full-dose BCG treatment or chemotherapy instillations for a maximum of one year is recommended. Conversely, full-dose intravesical BCG for one to three years is indicated in patients with high-risk tumors
[28].
Therapeutic options for patients with BCG-unresponsive disease include RC, further intravesical therapy, and systemic therapy. A relatively new addition to the landscape of treatment for BCG-unresponsive NMIBC is pembrolizumab. Initial results of the KEYNOTE-057 phase II trial were reported in February 2019 showing a 38.8% (40/102) complete response (CR) rate at 3 months. Following the presentation of these data, pembrolizumab received FDA approval in January 2020 for BCG-unresponsive high-risk NMIBC patients, ineligible for, or refusing RC. Key secondary endpoints were duration of response (DOR) and safety. At a median follow-up of 14 months, 72.5% of patients maintained CR, 25.0% experienced recurrent NMIBC after CR, but none progressed to MIBC. Treatment-related adverse events (AEs) occurred in 63.1% of patients, the most frequent being pruritus, fatigue, diarrhea, hypothyroidism, and maculopapular rash. Grade 3–4 AEs occurred in 12.6% of patients, and one death due to colitis was considered treatment-related
[29]. Updated data over a 2-year follow-up were submitted at the 2020 American Society of Clinical Oncology (ASCO) Annual Meeting. The median DOR was 16.2 months, and CR rate was 40.6% with 46.2% of responses longer than 12 months. The median PFS and OS were not reached
[30].
At the 2021 ASCO Genitourinary Cancers Symposium, Balar et al. reported additional results with an extended minimum follow-up of 26.3 months
[31]. Among those patients achieving CR, 33.3% remained in CR for ≥18 months and 23.1% for ≥24 months as of the data cutoff date. Of the 41.7% patients undergoing cystectomy after discontinuation of pembrolizumab, 35 (88%) had no pathological upstaging to MIBC, three (8%) had evidence of MIBC, and two (5%) had no available pathology data. Safety profile remained consistent with what had been previously reported.
Another phase II trial, SWOG S1605, tested atezolizumab in the same setting. The primary outcome was the pathological complete response (pCR) rate at six months, accomplished through mandatory biopsy. A pCR was observed in 30 (41.1%) patients at 3 months and in 19 (26.0%) at 6 months. The most common AEs were fatigue, pruritus, hypothyroidism, and nausea. Grade 3–5 AEs occurred in 12.3% of patients, and there was one treatment-related death due to myasthenia gravis
[32].
Several clinical trials with other ICI agents, both as monotherapy and as part of a combination therapy, are ongoing and in early-stage BC. Particularly relevant are the POTOMAC trial assessing durvalumab plus BCG in BCG-naïve patients, the KEYNOTE-676 study evaluating BCG-associated pembrolizumab in patients with recurrence after induction BCG therapy alone
[33], and the NCT03317158 trial establishing the safety of durvalumab as monotherapy and in combination with BCG and external beam radiation therapy (EBRT) in BCG-unresponsive NMIBC patients (
Table 2).
Table 2. Ongoing phase II/III trials with active recruitment on ICIs alone or in combination with chemotherapy in different settings of BC treatment.
| Trial |
Phase |
Allocation |
No. of Patients |
Study Populations |
Line of Treatment |
Experimental Arms |
Primary Outcome |
| NCT02736266 |
II |
N/A |
90 |
MIBC |
neoadjuvant prior to chemoradiation |
Pembrolizumab |
pCR |
| NCT02845323 |
II |
randomized |
44 |
MIBC |
neoadjuvant |
Nivolumab + Urelumab vs. Nivolumab |
Immune response (tumor infiltrating CD8+ T cell density) |
| NCT03520491 |
II |
not randomized |
45 |
Cisplatin-ineligible patients with MIBC |
neoadjuvant |
Nivolumab and Nivolumab + Ipilimumab |
No. of patients who proceed to RC-PLND |
| NCT03472274 |
II |
randomized |
99 |
BC patients |
neoadjuvant |
Durvalumab and Tremelimumab |
Antitumor activity |
| NCT03732677 |
III |
randomized |
1050 |
MIBC |
neoadjuvant/adjuvant |
Durvalumab + Gemcitabine + Cisplatin neoadjuvant treatment followed by Durvalumab alone for adjuvant treatment |
EFS |
| NCT04138628 |
II |
randomized |
282 |
Treatment of mBC at the time of biochemical relapse following RC |
adjuvant |
Atezolizumab |
CR |
| NCT03244384 |
III |
randomized |
739 |
Locally advanced and mUC |
adjuvant |
Pembrolizumab vs. observation |
OS, DFS |
| NCT04223856 |
III |
randomized |
760 |
Previously untreated locally advanced or mUC |
1st |
Enfortumab vedotin + Pembrolizumab vs. chemotherapy alone |
PFS, OS |
| NCT03036098 |
III |
randomized |
1290 |
Unresectable or mUC |
1st |
Nivolumab + Ipilimumab, or SoC chemotherapy vs. SoC Chemotherapy |
OS, PFS |
| NCT03682068 |
III |
randomized |
1434 |
Unresectable locally advanced or mUC |
1st |
Durvalumab + SoC chemotherapy and Durvalumab + Tremelimumab and SoC Chemotherapy vs. SoC chemotherapy alone |
OS |
| NCT03898180 |
III |
randomized |
694 |
Locally advanced or mUC |
1st |
Pembrolizumab + Lenvatinib vs. Pembrolizumab +placebo |
PFS, OS |
| NCT03697850 |
II |
randomized |
77 |
MIBC patients ineligible for RC |
maintenance therapy |
Atezolizumab |
DFS |