
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shangli Liu | -- | 2466 | 2023-06-15 11:14:08 | | | |
| 2 | Catherine Yang | Meta information modification | 2466 | 2023-06-16 03:28:18 | | |
Video Upload Options
On the basis of the verification of various laboratory simulations and field pilot production, academic and industrial circles have agreed on the technical feasibility of shale-oil exploitation after organic-rich shale in situ conversion. However, the kerogen cracking process is an endothermic reaction that needs to consume a certain amount of energy. In addition, during the heating process, inorganic minerals and water in the shale are heated synchronously and consume a significant amount of heat energy. Meanwhile, due to the long heating time, much of the heat energy of shale is also dissipated into the surrounding rock through heat conduction. In addition to the high heating costs, the required equipment for in situ conversion is more expensive. Therefore, many experts and companies are concerned about the economic feasibility of in situ conversion. The economic feasibility of oil shale in situ conversion depends on whether the energy consumption ratio (value of produced oil and gas products/(heating energy consumption cost + engineering costs)) is higher than 1. Only when the value of the produced petroleum products is significantly greater than the cost of heating energy consumption can it be expected to realize economic benefits through large-scale commercial development, the dilution of engineering, and other costs. Therefore, improving the ratio of the value of oil and gas products to the cost of heating energy consumption is very important.
1. Influence of Heating Mode
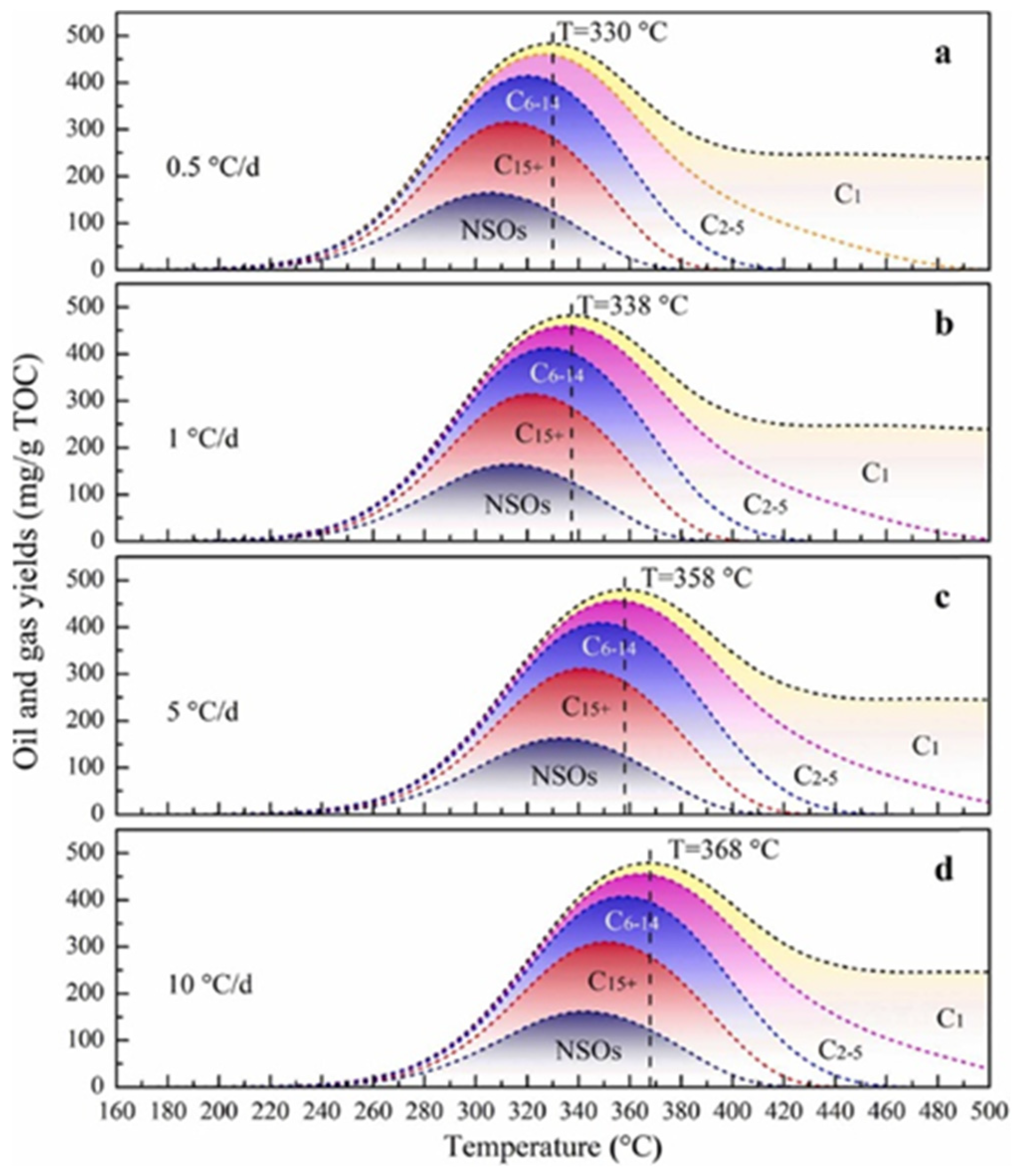
2. Influence of Formation Conditions
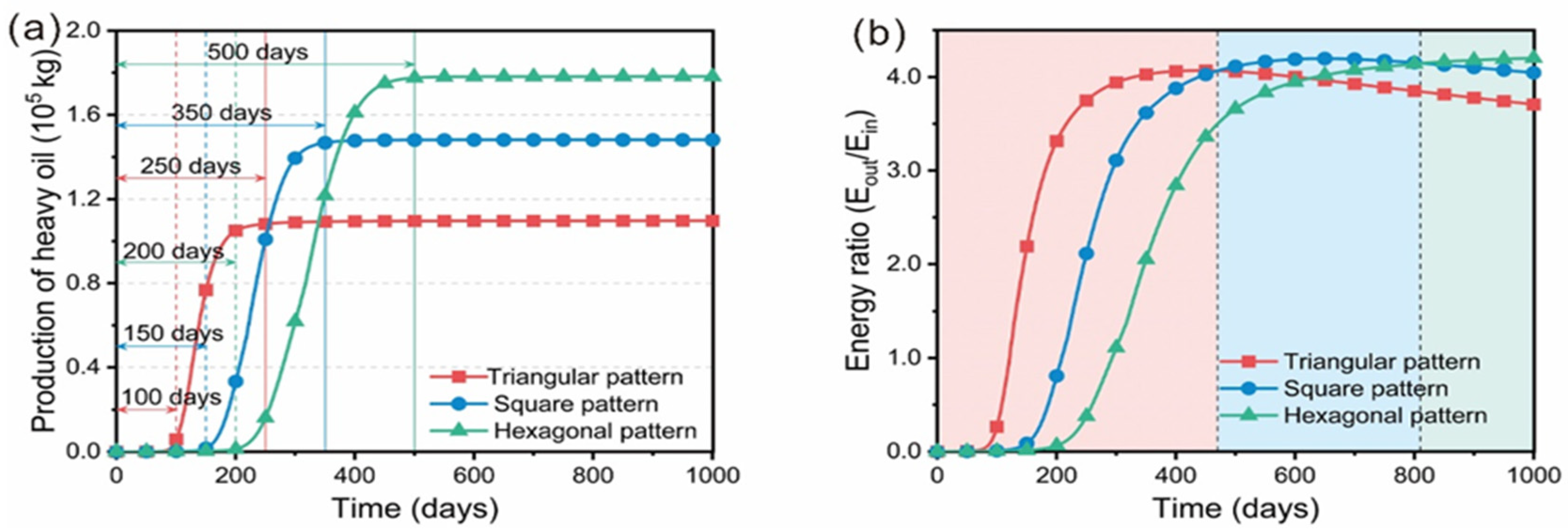
3. Distribution Pattern of Wells
4. Catalytic Agent
References
- Waples, D.W. The kinetics of in-reservoir oil destruction and gas formation: Constraints from experimental and empirical data, and from thermodynamics. Org. Geochem. 2000, 31, 553–575.
- Waples, D.W. Time and Temperature in Petroleum Formation—Application of Lopatins Method to Petroleum-Exploration. AAPG Bull. 1980, 64, 916–926.
- Tissot, B.; Durand, B.; Espitali, J.; Combaz, A. Influence of Nature and Diagenesis of Organic-Matter in Formation of Petroleum. AAPG Bull. 1974, 58, 499–506.
- Hou, D.; Feng, Z. Oil and Gas Geochemistry; Petroleum Industry Press: Beijing, China, 2011.
- Hou, L.; He, K.; Zhai, J.; Mi, J.; Weng, N. Compositional kinetics for hydrocarbon evolution in the pyrolysis of the Chang 7 organic matter: Implications for in-situ conversion of oil shale. J. Anal. Appl. Pyrolysis 2022, 162, 105434.
- Zhang, B.; Yu, C.; Cui, J.; Mi, J.; Li, H.; He, F. Kinetic simulation of hydrocarbon generation and its application to in-situ conversion of shale oil. Pet. Explor. Dev. 2019, 46, 1288–1296.
- Alhesan JS, A.; Fei, Y.; Marshall, M.; Jackson, W.R.; Qi, Y.; Chaffee, A.L.; Cassidy, P.J. Long time, low temperature pyrolysis of El-Lajjun oil shale. J. Anal. Appl. Pyrolysis 2018, 130, 135–141.
- Fan, Y.; Durlofsky, L.J.; Tchelepi, H.A. Numerical Simulation of the In-Situ Upgrading of Oil Shale. SPE J. 2010, 15, 368–381.
- Ma, Z.; Wang, Q.; Zheng, L.; Zhang, C. Identification method and application of temperature-time-conversion ratio for in-situ production of oil shale. J. Jilin Univ. Earth Sci. Ed. 2019, 49, 394–399.
- Song, D.; Wang, X.; Wu, C.; Meng, S.; Zhang, M.; Li, H.; Jiao, H.; Liu, X.; Jin, X.; Tuo, J. Petroleum Generation, Retention, and Expulsion in Lacustrine Shales Using an Artificial Thermal Maturation Approach: Implications for the In-Situ Conversion of Shale Oil. Energy Fuels 2021, 35, 358–373.
- Ma, Z.; Zheng, L.; Zhao, Z. Influence and its Revelation of oil shale in-situ Mining Simulation in different boundary conditions. J. Jilin Univ. Earth Sci. Ed. 2017, 47, 431–441.
- Xu, L.; Ma, Z.; Zheng, L.; Bao, F. Change of physical properties at different heating rates, time and water content for oil shale. Pet. Geol. Exp. 2018, 40, 545–550.
- Behar, F.; Lorant, F.; Lewan, M. Role of NSO compounds during primary cracking of a Type II kerogen and a Type III lignite. Org. Geochem. 2008, 39, 1–22.
- Dieckmann, V.; Schenk, H.J.; Horsfield, B.; Welte, D.H. Kinetics of petroleum generation and cracking by programmed-temperature closed-system pyrolysis of Toarcian Shales. Fuel 1998, 77, 23–31.
- Zhao, Q.; Dong, Y.; Zheng, L.; Xie, T.; Bawaa, B.; Jin, H.; Guo, L. Sub- and supercritical water conversion of organic-rich shale with low-maturity for oil and gas generation: Using Chang 7 shale as an example. Sustain. Energy Fuels 2022, 7, 155–163.
- Vakhin, A.V. Rock Mineral Components’ Effects on Heavy and Shale Oil Transformation during Aquathermolysis. Energies 2022, 15, 6047.
- Lewan, M.D. Sulphur-radical control on petroleum formation rates. Nature 1998, 391, 164–166.
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121.
- Zhao, F.; Li, B.; Che, D.; Liu, S. The mechanism of H2O in the superheated steam affecting the quality of in-situ pyrolysates of oil shale kerogen: Part B-favorable conversion of residues. Fuel 2023, 337, 127146.
- Lewan, M.D. Experiments on the role of water in petroleum formation. Geochim. Cosmochim. Acta 1997, 61, 3691–3723.
- Zhao, W.; Hu, S.; Hou, L. Connotation and strategic role of in-situ conversion processing of shale oil underground in the onshore China. Pet. Explor. Dev. 2018, 45, 563–572.
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Pet. Explor. Dev. 2020, 47, 877–887.
- Li, J.; Tang, D.; Xue, H.; Zheng, D.; Du, D. Discission of Oil Shale In-situ Conversion Process in China. J. Southwest Pet. Univ. 2014, 36, 58–64.
- Huang, H.; Yu, H.; Xu, W.; Lyu, C.; Micheal, M.; Xu, H.; Liu, H.; Wu, H. A coupled thermo-hydro-mechanical-chemical model for production performance of oil shale reservoirs during in-situ conversion process. Energy 2023, 268, 126700.
- Lu, S.; Wang, J.; Li, W.; Cao, Y.; Chen, F.; Li, J.; Xue, H.; Wang, M. In-situ upgrading and transformation of low-maturity shale: Economic feasibility and efficiency enhancement approaches from the perspective of energy consumption ratio. Earth Sci. Front. 2023, 30, 187.
- Song, R.; Meng, X.; Yu, C.; Bian, J.; Su, J. Oil shale in-situ upgrading with natural clay-based catalysts: Enhancement of oil yield and quality. Fuel 2022, 314, 123076.
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714.
- Meng, X.; Qi, Z.; Yu, C.; Bian, J.; Ma, Z.; Long, Q.; Su, J. Solid-Acid Catalytic Conversion of Oil Shale: Effects of Sulfonic Acid Grafting on Oil Yield Enhancing and Quality Improvement. ACS Omega 2021, 6, 5836–5845.
- Li, J.; Sun, X.; Wu, D.; Zhai, S.; Yang, S.; Toktonaliev, A.; Pan, Y. Application of Oil Shale Molecular Sieve Catalyst: A Review. Chem. Technol. Fuels Oils 2022, 58, 902–911.
- Chang, Z.B.; Chu, M.; Zhang, C.; Bai, S.X.; Lin, H.; Ma, L.B. Investigation of the Effect of Selected Transition Metal Salts on the Pyrolysis of Huadian Oil Shale, China. Oil Shale 2017, 34, 354–367.
- Yu, C.; Qi, Z.; Guo, Y.; Bian, J.; Meng, X.; Long, Q. Oil shale in situ catalytic conversion over clin/SBA-15 composites under subcritical water. J. Anal. Appl. Pyrolysis 2020, 152, 104942.
- Sun, J.; Hou, Q.; Lu, K.; Jin, J.; Guo, X.; Wang, J.; Huang, X.; Liao, B. Catalytic Pyrolysis of Oil Shale by CrCl3 and Its Molecular Simulation Mechanism. J. China Univ. Pet. Ed. Nat. Sci. 2023, 47, 74–80.
- Hu, S.; Xiao, C.; Liang, X.; Cao, Y.; Wang, X.; Li, M. The influence of oil shale in situ mining on groundwater environment: A water-rock interaction study. Chemosphere 2019, 228, 384–389.
- Taheri-Shakib, J.; Kantzas, A. A comprehensive review of microwave application on the oil shale: Prospects for shale oil production. Fuel 2021, 305, 121519.
- Wang, M.; Zhang, Y.; Li, J.; Ma, R.; Wang, X.; Li, Y.; Shao, H.; Zhang, J.; Fei, J.; Deng, Z.; et al. Thermal and nonthermal effect of microwave irradiation on the pore microstructure and hydrocarbon generation of organic matter in shale. Mar. Pet. Geol. 2023, 150, 106151.




