
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Young-Joon Kim | -- | 6725 | 2023-06-14 03:51:51 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 6728 | 2023-06-15 03:58:33 | | | | |
| 3 | Lindsay Dong | + 17 word(s) | 6745 | 2023-06-16 10:32:35 | | |
Video Upload Options
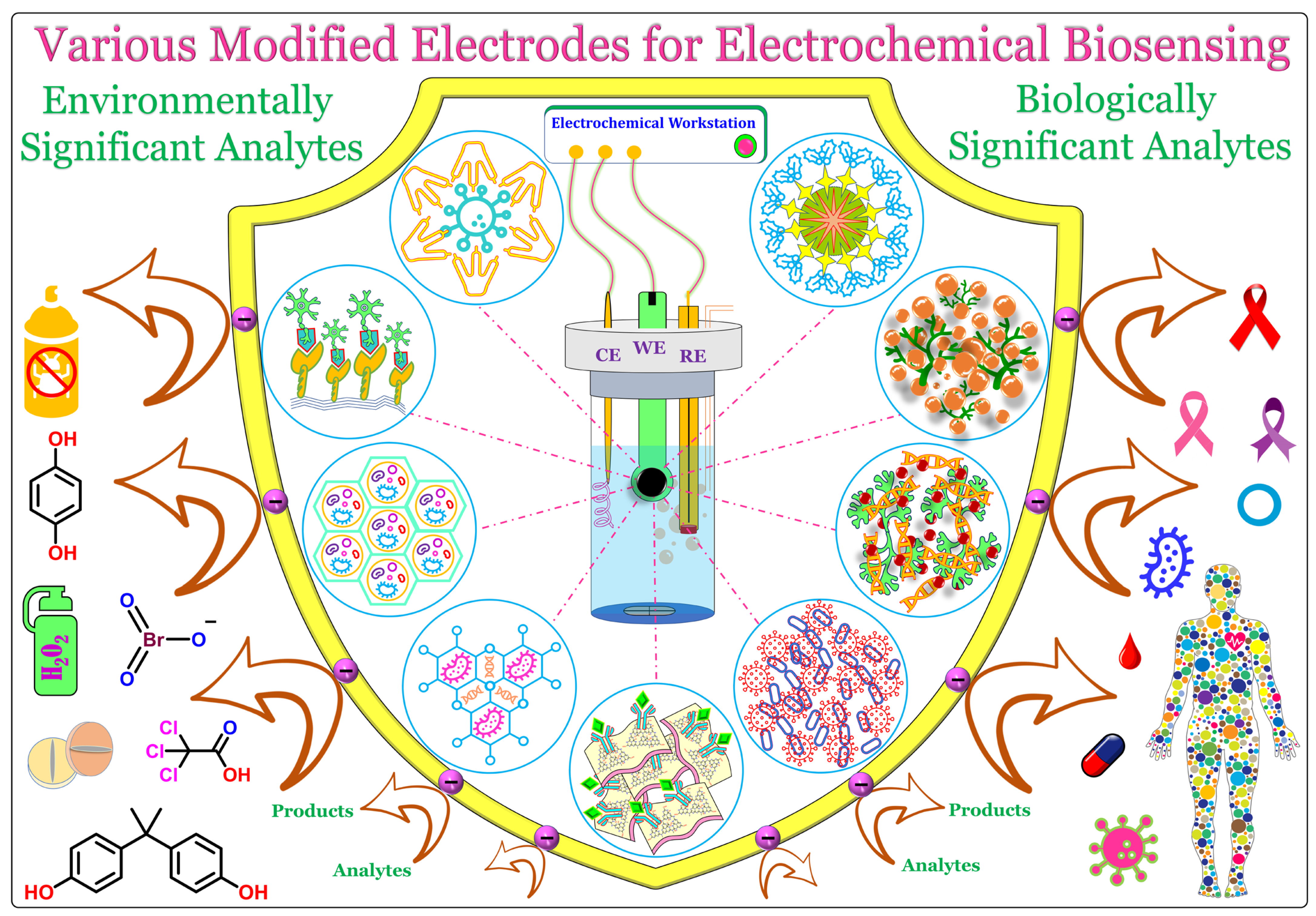
Electrochemical biosensors are superior technologies that are used to detect or sense biologically and environmentally significant analytes in a laboratory environment, or even in the form of portable handheld or wearable electronics.
1. Introduction
2. Various Nanomaterial-Modified Electrodes
2.1. Carbon-Based Nanomaterials
2.1.1. Carbon Black
2.1.2. Graphene and Its Derivatives
2.1.3. Carbon Nanotubes
2.2. Metal Nanomaterials
2.2.1. Metal Nanoparticles
2.2.2. Metal Oxide and Sulfide Nanoparticles
2.2.3. MXenes
2.3. Quantum Dots
2.4. Organic Frameworks
2.4.1. Metal Organic Frameworks
2.4.2. Covalent Organic Frameworks
2.5. Ionic Liquids
2.5.1. Functionalized ILs
2.5.2. ILs with Nanomaterials
3. Summary
Rational design of the sensor platform, followed by the sensible immobilization of biomolecules, are the crucial components in the fabrication of effectual electrochemical biosensors. The biomolecules are highly sensitive, and even a slight change in the microenvironment might result in denaturation and loss of biosensor performance [119][120]. The following are some of the key points that need to be kept in mind while designing biosensor platforms. The intended platform or the materials chosen for the fabrication of the biosensor platform should be biocompatible and nontoxic to biomolecules, since the biomolecules are made of polypeptide layers and the secondary and tertiary structures are held together by weak forces, such as hydrogen bonding, electrostatic, disulfide, and Van der Waals forces, which may unfold and disintegrate if the biocompatibility is not maintained. The second crucial factor is conductivity; the intended platform material should be highly conductive, since the active centers are deeply buried inside the thick, non-conducting polypeptide layers, and the material should be capable enough to connect to or communicate with the active centers. For instance, the FAD redox center in GOD, the iron center in Hb, and Cyt-c are all deeply buried inside the protein layers and in order to realize its activity, the platform should communicate the active centers without damaging the secondary and tertiary structures [121][122]. The third important factor is the method of coating or immobilization of the biomolecules on the modified electrode surface. Some of the commonly used strategies are physical mixing or composite formation; drop casting (adsorption); dip coating; entrapping or encapsulating within the pores, cavities, or covers with polymer membranes such as Nafion, etc.; covalent bonding, cross-coupling, SAM and so forth. If the biomolecules are physically held on the surface by adsorption or drop casting, there is a possibility that the biomolecules may leach out during potential cycling. On the other hand, entrapment or encapsulation of biomolecules within the porous network may retain the biomolecules more effectively than adsorption and physical mixing, but they may not involve actively, due to the improper orientation of biomolecules. Therefore, the orientation of the immobilized biomolecules also needs to be maintained in order to display an effective performance. Covalent binding via a bifunctional linker and SAM formation directed by specific functional groups may produce more stable and effective biosensors, since the biomolecules are coupled to the platform strongly and uniform orientations are maintained properly. Moreover, direct covalent immobilization is preferred compared to other methods, since it does not require a bifunctional linker or other external components, such as polymers or Nafion, in order to hold the biomolecules on the substrate. Furthermore, the stability of the fabricated platform plays a significant role in the performance of the developed biosensor, and its stability depends on how well the enzymes are immobilized on the biosensor platform. Therefore, a stable and effective immobilization of biomolecules improves the shelf life and performance of fabricated biosensors. Moreover, the developed platform should have a large surface area, so that more biomolecules could be accommodated on its surface. Finally, the reaction and storage conditions also matter and determine the stability and functioning of the biosensor. For instance, if the pH of the electrolyte solution is too low or too high, it might inhibit the biomolecules’ activity and result in poor performance. Similarly, if the temperature and/or the electrochemical reaction potential is too high, it would denature the biomolecule permanently and result in the failure of the biosensor. Therefore, in order to fabricate effectual biosensors, it is necessary to be prudent and pay attention to the aforementioned requisites.
References
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H.; et al. Graphene Biodevices for Early Disease Diagnosis Based on Biomarker Detection. ACS Sens. 2021, 6, 3841–3881.
- Parsa, S.F.; Vafajoo, A.; Rostami, A.; Salarian, R.; Rabiee, M.; Rabiee, N.; Rabiee, G.; Tahriri, M.; Yadegari, A.; Vashaee, D.; et al. Early Diagnosis of Disease Using Microbead Array Technology: A Review. Anal. Chim. Acta 2018, 1032, 1–17.
- Ha, M.; Lim, S.; Ko, H. Wearable and Flexible Sensors for User-Interactive Health-Monitoring Devices. J. Mater. Chem. B 2018, 6, 4043–4064.
- Pawlik, P.; Błochowiak, K. The Role of Salivary Biomarkers in the Early Diagnosis of Alzheimer’s Disease and Parkinson’s Disease. Diagnostics 2021, 11, 371.
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572.
- Lee, Y.H.; Kweon, O.Y.; Kim, H.; Yoo, J.H.; Han, S.G.; Oh, J.H. Recent Advances in Organic Sensors for Health Self-Monitoring Systems. J. Mater. Chem. C 2018, 6, 8569–8612.
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386.
- Ma, S.; Lin, M.; Tang, J.; Liu, R.; Yang, Y.; Yu, Y.; Li, G.; An, T. Occurrence and Fate of Polycyclic Aromatic Hydrocarbons from Electronic Waste Dismantling Activities: A Critical Review from Environmental Pollution to Human Health. J. Hazard. Mater. 2022, 424, 127683.
- Chormare, R.; Kumar, M.A. Environmental Health and Risk Assessment Metrics with Special Mention to Biotransfer, Bioaccumulation and Biomagnification of Environmental Pollutants. Chemosphere 2022, 302, 134836.
- Jarin, M.; Dou, Z.; Gao, H.; Chen, Y.; Xie, X. Salinity Exchange between Seawater/Brackish Water and Domestic Wastewater through Electrodialysis for Potable Water. Front. Environ. Sci. Eng. 2023, 17, 16.
- Stefan, D.S.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 14, 3939.
- Kanwal, Q.; Li, J.; Zeng, X. Mapping Recyclability of Industrial Waste for Anthropogenic Circularity: A Circular Economy Approach. ACS Sustain. Chem. Eng. 2021, 9, 11927–11936.
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536.
- Cai, Y.; Ling, Q.; Yi, Y.; Chen, Z.; Yang, H.; Hu, B.; Liang, L.; Wang, X. Application of Covalent Organic Frameworks in Environmental Pollution Management. Appl. Catal. A 2022, 643, 118733.
- Mahamood, M.; Khan, F.R.; Zahir, F.; Javed, M.; Alhewairini, S.S. Bagarius Bagarius, and Eichhornia Crassipes Are Suitable Bioindicators of Heavy Metal Pollution, Toxicity, and Risk Assessment. Sci. Rep. 2023, 13, 1824.
- Verma, N.; Velmurugan, G.V.; Winford, E.; Coburn, H.; Kotiya, D.; Leibold, N.; Radulescu, L.; Despa, S.; Chen, K.C.; van Eldik, L.J.; et al. Aβ Efflux Impairment and Inflammation Linked to Cerebrovascular Accumulation of Amyloid-Forming Amylin Secreted from Pancreas. Commun. Biol. 2023, 6, 2.
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid Detection of Apple Juice Concentrate Adulteration with Date Concentrate, Fructose and Glucose Syrup Using HPLC-RID Incorporated with Chemometric Tools. Food Chem. 2022, 370, 131015.
- Wang, X.Y.; Zhu, G.B.; Cao, W.D.; Liu, Z.J.; Pan, C.G.; Hu, W.J.; Zhao, W.Y.; Sun, J.F. A Novel Ratiometric Fluorescent Probe for the Detection of Uric Acid in Human Blood Based on H2O2-Mediated Fluorescence Quenching of Gold/Silver Nanoclusters. Talanta 2019, 191, 46–53.
- Kim, T.; Lee, J.; Lee, J.P.; Kim, B.N.; Kim, Y.H.; Lee, Y.S.; Min, J. Screening of Novel Peptides That Specifically Interact with Vitamin D Bound Biocomplex Proteins. Sci. Rep. 2023, 13, 2116.
- Celik, C.; Demir, N.Y.; Duman, M.; Ildiz, N.; Ocsoy, I. Red Cabbage Extract-Mediated Colorimetric Sensor for Swift, Sensitive and Economic Detection of Urease-Positive Bacteria by Naked Eye and Smartphone Platform. Sci. Rep. 2023, 13, 2056.
- Nukazuka, A.; Asai, S.; Hayakawa, K.; Nakagawa, K.; Kanazashi, M.; Kakizoe, H.; Hayashi, K.; Kawahara, T.; Sawada, K.; Kuno, H.; et al. Electrical Biosensing System Utilizing Ion-Producing Enzymes Conjugated with Aptamers for the Sensing of Severe Acute Respiratory Syndrome Coronavirus 2. Sens. Bio-Sens. Res. 2023, 39, 100549.
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Ţălu, Ş.; Trejo, G.; Méndez-Albores, A. Electrochemical Biosensor for Sensitive Quantification of Glyphosate in Maize Kernels. Electroanalysis 2019, 31, 927–935.
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical Performance of an Antibody-Free Assay for Plasma Aβ42/Aβ40 to Detect Early Alterations of Alzheimer’s Disease in Individuals with Subjective Cognitive Decline. Alzheimers Res. Ther. 2023, 15, 2.
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In Vivo Electrochemical Biosensors: Recent Advances in Molecular Design, Electrode Materials, and Electrochemical Devices. Anal. Chem. 2023, 95, 388–406.
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-Derivative in a Tyrosinase-Based Amperometric Biosensor for Epinephrine Detection. Electroanalysis 2021, 33, 1639–1645.
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent Advances in Hydroxyapatite-Based Electrochemical Biosensors: Applications and Future Perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542.
- Laraib, U.; Sargazi, S.; Rahdar, A.; Khatami, M.; Pandey, S. Nanotechnology-Based Approaches for Effective Detection of Tumor Markers: A Comprehensive State-of-the-Art Review. Int. J. Biol. Macromol. 2022, 195, 356–383.
- Koo, K.M.; Kim, C.D.; Ju, F.N.; Kim, H.; Kim, C.H.; Kim, T.H. Recent Advances in Electrochemical Biosensors for Monitoring Animal Cell Function and Viability. Biosensors 2022, 12, 1162.
- Li, H.; Qi, H.; Chang, J.; Gai, P.; Li, F. Recent Progress in Homogeneous Electrochemical Sensors and Their Designs and Applications. TrAC Trends Anal. Chem. 2022, 156, 116712.
- Falina, S.; Anuar, K.; Shafiee, S.A.; Juan, J.C.; Manaf, A.A.; Kawarada, H.; Syamsul, M. Two-Dimensional Non-Carbon Materials-Based Electrochemical Printed Sensors: An Updated Review. Sensors 2022, 22, 9358.
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical Sensors Based on Molecularly Imprinted Polymer on Nanostructured Carbon Materials: A Review. J. Electroanal. Chem. 2019, 840, 343–366.
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (Bio) Sensors Go Green. Biosens. Bioelectron. 2020, 163, 112270.
- Zhang, F.T.; Cai, L.Y.; Zhou, Y.L.; Zhang, X.X. Immobilization-Free DNA-Based Homogeneous Electrochemical Biosensors. TrAC Trends Anal. Chem. 2016, 85, 17–32.
- Sheikholeslam, M.; Nanda, P.; Sanati, A.; Pritzker, M.; Chen, P. Direct Electrochemistry of Hemoglobin/Peptide-Carbon Nanotube Modified Electrode for Hydrogen Peroxide Biosensing. Mater. Lett. 2023, 335, 133799.
- Lawal, A.T. Progress in Utilisation of Graphene for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 106, 149–178.
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A Novel Nitrite Biosensor Based on the Direct Electrochemistry of Hemoglobin Immobilized on MXene-Ti3C2. Sens. Actuators B 2015, 218, 60–66.
- Sha, H. Direct Electrochemistry of Hemoglobin on Electrodeposited Three-Dimensional Interconnected Graphene-Silver Nanocomposite Modified Electrode. Int. J. Electrochem. Sci. 2016, 11, 9656–9665.
- Zhang, Y.; Li, N.; Xu, Y.; Liu, X.; Ma, Y.; Huang, Z.; Luo, H.; Hou, C.; Huo, D. A Novel Electrochemical Biosensor Based on Nano Composite Material for Ultrasensitive Detection of HER2. Bioelectrochemistry 2023, 150, 108362.
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent Electrochemical Sensors and Biosensors for Toxic Agents Based on Screen-Printed Electrodes Equipped with Nanomaterials. Microchem. J. 2023, 185, 108281.
- Tseng, W.T.; Chou, Y.Y.; Wu, J.G.; Wang, Y.C.; Tseng, T.N.; Pan, S.W.; Luo, S.C.; Ho, M.L. An Electrochemical Conducting Polymer-Based Biosensor for Leukocyte Esterase and Nitrite Detection for Diagnosing Urinary Tract Infections: A Pilot Study. Microchem. J. 2023, 188, 108493.
- Ferreira, L.M.C.; Silva, P.S.; Augusto, K.K.L.; Gomes-Júnior, P.C.; Farra, S.O.D.; Silva, T.A.; Fatibello-Filho, O.; Vicentini, F.C. Using Nanostructured Carbon Black-Based Electrochemical (Bio)Sensors for Pharmaceutical and Biomedical Analyses: A Comprehensive Review. J. Pharm. Biomed. Anal. 2022, 221, 115032.
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of Carbon Black Functionalization on the Analytical Performance of a Tyrosinase Biosensor Based on Glassy Carbon Electrode Modified with Dihexadecylphosphate Film. Enzyme Microb. Technol. 2018, 116, 41–47.
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a Phage-Based Electrochemical Biosensor for Detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345.
- Melios, N.; Tsouti, V.; Chatzandroulis, S.; Tsekenis, G. Development of an All-Carbon Electrochemical Biosensor on a Flexible Substrate for the Sensitive Detection of Glucose. Eng. Proc. 2022, 16, 17.
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-Based Magneto-Immunosensor on Carbon Black Modified Screen-Printed Electrodes for Point-of-Need Detection of Aflatoxin B1 in Cereals. Anal. Chim. Acta 2022, 1221, 340118.
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond Graphene: Electrochemical Sensors and Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2017, 89, 152–166.
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene Nanomaterials: The Wondering Material from Synthesis to Applications. Sens. Int. 2022, 3, 100190.
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895.
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous Capillary-Flow Sensing of Glucose and Lactate in Sweat with an Electrochemical Sensor Based on Functionalized Graphene Oxide. Sens. Actuators B 2021, 344, 130253.
- Elshafey, R.; Abo-Sobehy, G.F.; Radi, A.E. Graphene Oxide/Graphene Quantum Dots: A Platform for Probing Ds-DNA-Dimethoate Interaction and Dimethoate Sensing. J. Electroanal. Chem. 2021, 899, 115678.
- Saleem, S.J.; Guler, M. Electroanalytical Determination of Paracetamol Using Pd Nanoparticles Deposited on Carboxylated Graphene Oxide Modified Glassy Carbon Electrode. Electroanalysis 2019, 31, 2187–2198.
- Erden, P.E.; Erdoğan, Z.Ö.; Öztürk, F.; Koçoğlu, İ.O.; Kılıç, E. Amperometric Biosensors for Tyramine Determination Based on Graphene Oxide and Polyvinylferrocene Modified Screen-Printed Electrodes. Electroanalysis 2019, 31, 2368–2378.
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486.
- Mujica, M.L.; Tamborelli, A.; Vaschetti, V.M.; Espinoza, L.C.; Bollo, S.; Dalmasso, P.R.; Rivas, G.A. Two Birds with One Stone: Integrating Exfoliation and Immunoaffinity Properties in Multi-Walled Carbon Nanotubes by Non-Covalent Functionalization with Human Immunoglobulin G. Microchim. Acta 2023, 190, 73.
- González-Hernández, J.; Moya-Alvarado, G.; Alvarado-Gámez, A.L.; Urcuyo, R.; Barquero-Quirós, M.; Arcos-Martínez, M.J. Electrochemical Biosensor for Quantitative Determination of Fentanyl Based on Immobilized Cytochrome c on Multi-Walled Carbon Nanotubes Modified Screen-Printed Carbon Electrodes. Microchim. Acta 2022, 189, 483.
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical Biosensors Based on in Situ Grown Carbon Nanotubes on Gold Microelectrode Array Fabricated on Glass Substrate for Glucose Determination. Microchim. Acta 2023, 190, 55.
- Alvarez-Paguay, J.; Fernández, L.; Bolaños-Méndez, D.; González, G.; Espinoza-Montero, P.J. Evaluation of an Electrochemical Biosensor Based on Carbon Nanotubes, Hydroxyapatite and Horseradish Peroxidase for the Detection of Hydrogen Peroxide. Sens. Bio-Sens. Res. 2022, 37, 100514.
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase Bioconjugate and Multi-Walled Carbon Nanotubes-Based Biosensor for Bisphenol A Analysis. Bioelectrochemistry 2022, 144, 108033.
- Yang, M.; Wang, H.; Liu, P.; Cheng, J. A 3D Electrochemical Biosensor Based on Super-Aligned Carbon NanoTube Array for Point-of-Care Uric Acid Monitoring. Biosens. Bioelectron. 2021, 179, 113082.
- Lu, C.; Zhou, S.; Gao, F.; Lin, J.; Liu, J.; Zheng, J. DNA-Mediated Growth of Noble Metal Nanomaterials for Biosensing Applications. TrAC Trends Anal. Chem. 2022, 148, 116533.
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131.
- Thao, D.V.P.; Thuy Ngan, D.T.; Tuan, D.V.; Lan, H.; Thi Nguyet, N.; Thu, V.V.; Hung, V.P.; Dinh Tam, P. Facile Preparation of Copper Nanoparticles in Environmentally Friendly Solvent for DNA Sensor Application. Mater. Today Commun. 2022, 33, 104161.
- Castro, A.C.H.; Bezerra, Í.R.S.; Pascon, A.M.; da Silva, G.H.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular Label-Free Electrochemical Biosensor Loading Nature-Inspired Peptide toward the Widespread Use of COVID-19 Antibody Tests. ACS Nano 2022, 16, 14239–14253.
- Medrades, J.d.P.; Maciel, C.C.; Moraes, A.d.S.; Leite, F.d.L.; Ferreira, M. Flavin Adenine Dinucleotide Functionalized Gold Nanoparticles for the Electrochemical Detection of Dopamine. Sens. Actuators Rep. 2022, 4, 100085.
- Rawat, C.S.; Bagaria, A. Fabrication and Characterization of an Electrochemical Glucose Biosensor Using Zinc Oxide Nanoparticles and Soy Protein Isolate Film Support Matrix. MRS Commun. 2022, 12, 930–936.
- Yin, Z.; Ji, Z.; Bloom, B.P.; Jayapalan, A.; Liu, M.; Zeng, X.; Waldeck, D.H.; Wei, J. Manipulating Cobalt Oxide on N-Doped Aligned Electrospun Carbon Nanofibers towards Instant Electrochemical Detection of Dopamine Secreted by Living Cells. Appl. Surf. Sci. 2022, 577, 151912.
- Hilal, M.; Xie, W.; Yang, W. Straw-Sheaf–like Co3O4 for Preparation of an Electrochemical Non-Enzymatic Glucose Sensor. Microchim. Acta 2022, 189, 364.
- Centane, S.; Mgidlana, S.; Openda, Y.; Nyokong, T. Electrochemical Detection of Human Epidermal Growth Factor Receptor 2 Using an Aptamer on Cobalt Phthalocyanines—Cerium Oxide Nanoparticle Conjugate. Bioelectrochemistry 2022, 146, 108146.
- Tri Murti, B.; Huang, Y.J.; Darumas Putri, A.; Lee, C.P.; Hsieh, C.M.; Wei, S.M.; Tsai, M.L.; Peng, C.W.; Yang, P.K. Free-Standing Vertically Aligned Tin Disulfide Nanosheets for Ultrasensitive Aptasensor Design toward Alzheimer’s Diagnosis Applications. Chem. Eng. J. 2023, 452, 139394.
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled Carbon Nanotubes Coated with Cobalt(II) Sulfide Nanoparticles for Electrochemical Sensing of Glucose via Direct Electron Transfer to Glucose Oxidase. Microchim. Acta 2020, 187, 80.
- Rohaizad, N.; Mayorga-Martinez, C.C.; Sofer, Z.; Webster, R.D.; Pumera, M. Niobium-Doped TiS2: Formation of TiS3 Nanobelts and Their Effects in Enzymatic Biosensors. Biosens. Bioelectron. 2020, 155, 112114.
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical MoS2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 197, 113797.
- Li, W.; Zhao, Z.; Yang, W.; Su, Q.; Na, C.; Zhang, X.; Zhao, R.; Song, H. Immobilization of Bovine Hemoglobin on Au Nanoparticles/MoS2 Nanosheets—Chitosan Modified Screen-Printed Electrode as Chlorpyrifos Biosensor. Enzyme Microb. Technol. 2022, 154, 109959.
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185.
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851.
- Mathew, M.; Rout, C.S. Electrochemical Biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782.
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An Organ-Like Titanium Carbide Material (MXene) with Multilayer Structure Encapsulating Hemoglobin for a Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B16–B21.
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D Transition Metal Carbide MXene as a Robust Biosensing Platform for Enzyme Immobilization and Ultrasensitive Detection of Phenol. Biosens. Bioelectron. 2018, 107, 69–75.
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A Label-Free Electrochemical Biosensor for Highly Sensitive Detection of Gliotoxin Based on DNA Nanostructure/MXene Nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531.
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828.
- Theyagarajan, K.; Aayushi, P.S.; Devadoss, A.; Thenmozhi, K.; Senthilkumar, S. Chapter 11. Quantum Dots-Based Disposable Sensors. In Disposable Electrochemical Sensors for Healthcare Monitoring: Material Properties and Design; Pandikumar, A., Devi, K.S.S., Eds.; Royal Society of Chemistry: London, UK, 2021; pp. 314–352.
- Taghavi, F.; Moeinpour, F.; Khojastehnezhad, A.; Abnous, K.; Taghdisi, S.M. Recent Applications of Quantum Dots in Optical and Electrochemical Aptasensing Detection of Lysozyme. Anal. Biochem. 2021, 630, 114334.
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene Quantum Dots-Based Electrochemical Biosensor for Catecholamine Neurotransmitters Detection. Electroanalysis 2018, 30, 1781–1790.
- Fatima, B.; Hussain, D.; Bashir, S.; Hussain, H.T.; Aslam, R.; Nawaz, R.; Rashid, H.N.; Bashir, N.; Majeed, S.; Ashiq, M.N.; et al. Catalase Immobilized Antimonene Quantum Dots Used as an Electrochemical Biosensor for Quantitative Determination of H2O2 from CA-125 Diagnosed Ovarian Cancer Samples. Mater. Sci. Eng. C 2020, 117, 111296.
- Zhao, Y.; Huang, J.; Huang, Q.; Tao, Y.; Gu, R.; Li, H.-Y.; Liu, H. Electrochemical Biosensor Employing PbS Colloidal Quantum Dots/Au Nanospheres-Modified Electrode for Ultrasensitive Glucose Detection. Nano Res. 2022.
- Safitri, E.; Heng, L.Y.; Ahmad, M.; Tan, L.L.; Nazaruddin, N.; Suhud, K.; Chiang, C.P.; Iqhrammullah, M. Electrochemical DNA Biosensor Based on Mercaptopropionic Acid-Capped ZnS Quantum Dots for Determination of the Gender of Arowana Fish. Biosensors 2022, 12, 650.
- Moazampour, M.; Zare, H.R.; Shekari, Z. Femtomolar Determination of an Ovarian Cancer Biomarker (MiR-200a) in Blood Plasma Using a Label Free Electrochemical Biosensor Based on L-Cysteine Functionalized ZnS Quantum Dots. Anal. Methods 2021, 13, 2021–2029.
- Richard, Y.A.; Lincy, S.A.; Piraman, S.; Dharuman, V. Label-Free Electrochemical Detection of Cancer Biomarkers DNA and Anti-P53 at Tin Oxide Quantum Dot-Gold-DNA Nanoparticle Modified Electrode. Bioelectrochemistry 2023, 150, 108371.
- Majumdar, S.; Moosavi, S.M.; Jablonka, K.M.; Ongari, D.; Smit, B. Diversifying Databases of Metal Organic Frameworks for High-Throughput Computational Screening. ACS Appl. Mater. Interfaces 2021, 13, 61004–61014.
- Yan, L.; Gopal, A.; Kashif, S.; Hazelton, P.; Lan, M.; Zhang, W.; Chen, X. Metal Organic Frameworks for Antibacterial Applications. Chem. Eng. J. 2022, 435, 134975.
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646.
- Luo, G. Electrochemical Myoglobin Biosensor Based on Magnesium Metal-Organic Frameworks and Gold Nanoparticles Composite Modified Electrode. Int. J. Electrochem. Sci. 2019, 14, 2405–2413.
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ni, G.; He, F.; Li, W.; Ming, D. Molybdenum Disulfide Supported on Metal-Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Appl. Bio Mater. 2021, 4, 5494–5502.
- Xue, Y.; Wang, Y.; Feng, S.; Yan, M.; Huang, J.; Yang, X. A Dual-Amplification Mode and Cu-Based Metal-Organic Frameworks Mediated Electrochemical Biosensor for Sensitive Detection of MicroRNA. Biosens. Bioelectron. 2022, 202, 113992.
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic Acid-Functionalized Zirconium Metal-Organic Frameworks Based Electrochemical Impedance Biosensor for the Cancer Cell Detection. Sens. Actuators B 2019, 301, 127073.
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework-Based Homogeneous Electrochemical Biosensor for Simultaneous Detection of Multiple Tumor Biomarkers. Anal. Chem. 2019, 91, 3604–3610.
- Wang, L.; Meng, T.; Liang, L.; Sun, J.; Wu, S.; Wang, H.; Yang, X.; Zhang, Y. Fabrication of Amine-Functionalized Metal-Organic Frameworks with Embedded Palladium Nanoparticles for Highly Sensitive Electrochemical Detection of Telomerase Activity. Sens. Actuators B 2019, 278, 133–139.
- Dong, P.; Zhu, L.; Huang, J.; Ren, J.; Lei, J. Electrocatalysis of Cerium Metal-Organic Frameworks for Ratiometric Electrochemical Detection of Telomerase Activity. Biosens. Bioelectron. 2019, 138, 111313.
- Huang, S.; Lu, M.; Wang, L. Cytochrome C-Multiwalled Carbon Nanotube and Cobalt Metal Organic Framework/Gold Nanoparticle Immobilized Electrochemical Biosensor for Nitrite Detection. RSC Adv. 2020, 11, 501–509.
- Martínez-Periñán, E.; Martínez-Fernández, M.; Segura, J.L.; Lorenzo, E. Electrochemical (Bio)Sensors Based on Covalent Organic Frameworks (COFs). Sensors 2022, 22, 4758.
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Zhao, F.; Liu, H.; Mathe, S.D.R.; Dong, A.; Zhang, J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2018, 8, 15.
- Yildirim, O.; Derkus, B. Triazine-Based 2D Covalent Organic Frameworks Improve the Electrochemical Performance of Enzymatic Biosensors. J. Mater. Sci. 2020, 55, 3034–3044.
- Xiao, Y.; Wu, N.; Wang, L.; Chen, L. A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors 2022, 12, 899.
- Sun, X.; Xie, Y.; Chu, H.; Long, M.; Zhang, M.; Wang, Y.; Hu, X. A Highly Sensitive Electrochemical Biosensor for the Detection of Hydroquinone Based on a Magnetic Covalent Organic Framework and Enzyme for Signal Amplification. New J. Chem. 2022, 46, 11902–11909.
- Li, H.; Kou, B.; Yuan, Y.; Chai, Y.; Yuan, R. Porous Fe3O4@COF-Immobilized Gold Nanoparticles with Excellent Catalytic Performance for Sensitive Electrochemical Detection of ATP. Biosens. Bioelectron. 2022, 197, 113758.
- Han, Y.; Lu, J.; Wang, M.; Sun, C.; Yang, J.; Li, G. An Electrochemical Biosensor for Exosome Detection Based on Covalent Organic Frameworks Conjugated with DNA and Horseradish Peroxidase. J. Electroanal. Chem. 2022, 920, 116576.
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Chaurasia, J.P.; Kumar Srivastava, A. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414.
- Cetinkaya, A.; Kaya, S.I.; Yence, M.; Budak, F.; Ozkan, S.A. Ionic Liquid-Based Materials for Electrochemical Sensor Applications in Environmental Samples. Trends Environ. Anal. Chem. 2023, 37, e00188.
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical Impedimetric Biosensors, Featuring the Use of Room Temperature Ionic Liquids (RTILs): Special Focus on Non-Faradaic Sensing. Biosens. Bioelectron. 2021, 177, 112940.
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of Ionic Liquids towards Protein Stability: A Comprehensive Overview on the Current Understanding and Their Implications. Int. J. Biol. Macromol. 2017, 96, 611–651.
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812.
- Wang, X.; Hao, J. Recent Advances in Ionic Liquid-Based Electrochemical Biosensors. Sci. Bull. 2016, 61, 1281–1295.
- Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Rationally Designed Naphthyl Substituted Amine Functionalized Ionic Liquid Platform for Covalent Immobilization and Direct Electrochemistry of Hemoglobin. Sci. Rep. 2019, 9, 10428.
- Manoj, D.; Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Aldehyde Functionalized Ionic Liquid on Electrochemically Reduced Graphene Oxide as a Versatile Platform for Covalent Immobilization of Biomolecules and Biosensing. Biosens. Bioelectron. 2018, 103, 104–112.
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly Imprinted Electrochemical Biosensor Based on Chitosan/Ionic Liquid-Graphene Composites Modified Electrode for Determination of Bovine Serum Albumin. Sens. Actuators B 2016, 225, 305–311.
- Niamsi, W.; Larpant, N.; Kalambate, P.K.; Primpray, V.; Karuwan, C.; Rodthongkum, N.; Laiwattanapaisal, W. Paper-Based Screen-Printed Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites Enabled Electrochemical Detection for Glucose Sensing. Biosensors 2022, 12, 852.
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzyme Microb. Technol. 2022, 156, 110006.
- Bisht, N.; Dwivedi, N.; Khosla, A.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Review—Recent Advances in Polydopamine-Based Electrochemical Biosensors. J. Electrochem. Soc. 2022, 169, 107505.
- Chen, J.; Nilghaz, A.; Chen, X.; Liu, S.; Tian, J. Direct Electrochemical Detection of Glucose on PEDOT Functionalized Screen-Printed Electrodes. J. Electrochem. Soc. 2022, 169, 087502.
- Shen, Y.; Qian, X.; Mi, X.; Tu, Y. An Ultrasensitive Electrochemiluminescent Sensing Platform for Oxygen Metabolism Based on Bioactive Magnetic Beads. Bioelectrochemistry 2022, 145, 108086.





