Electrochemical biosensors are superior technologies that are used to detect or sense biologically and environmentally significant analytes in a laboratory environment, or even in the form of portable handheld or wearable electronics.
- modified electrode
- fabrication strategy
- electrochemical immunosensor
- aptasensor
- sensor
1. Introduction
2. Various Nanomaterial-Modified Electrodes
Nanomaterials (NMs) are materials ranging in size from 1 to100 nm. NMs have superior optical, physical, chemical, and electrochemical properties compared to their bulk. These properties can be tuned or tailored by altering the shape, size, and constituents of NMs. The following are some recently employed NMs and their hybrids in the design and fabrication of electrochemical biosensors. Figure 1 shows the general representation of a common three-electrode system, comprising a working electrode (WE), reference electrode (RE), and counter electrode (CE), and a graphical illustration of various biosensors fabricated using different electrode materials (functionalized carbons and dendrimers, quantum dots and metal nanoparticles-decorated 2D materials, metal and covalent organic frameworks, porous materials, and functionalized ionic liquids) for the biosensing of significant analytes.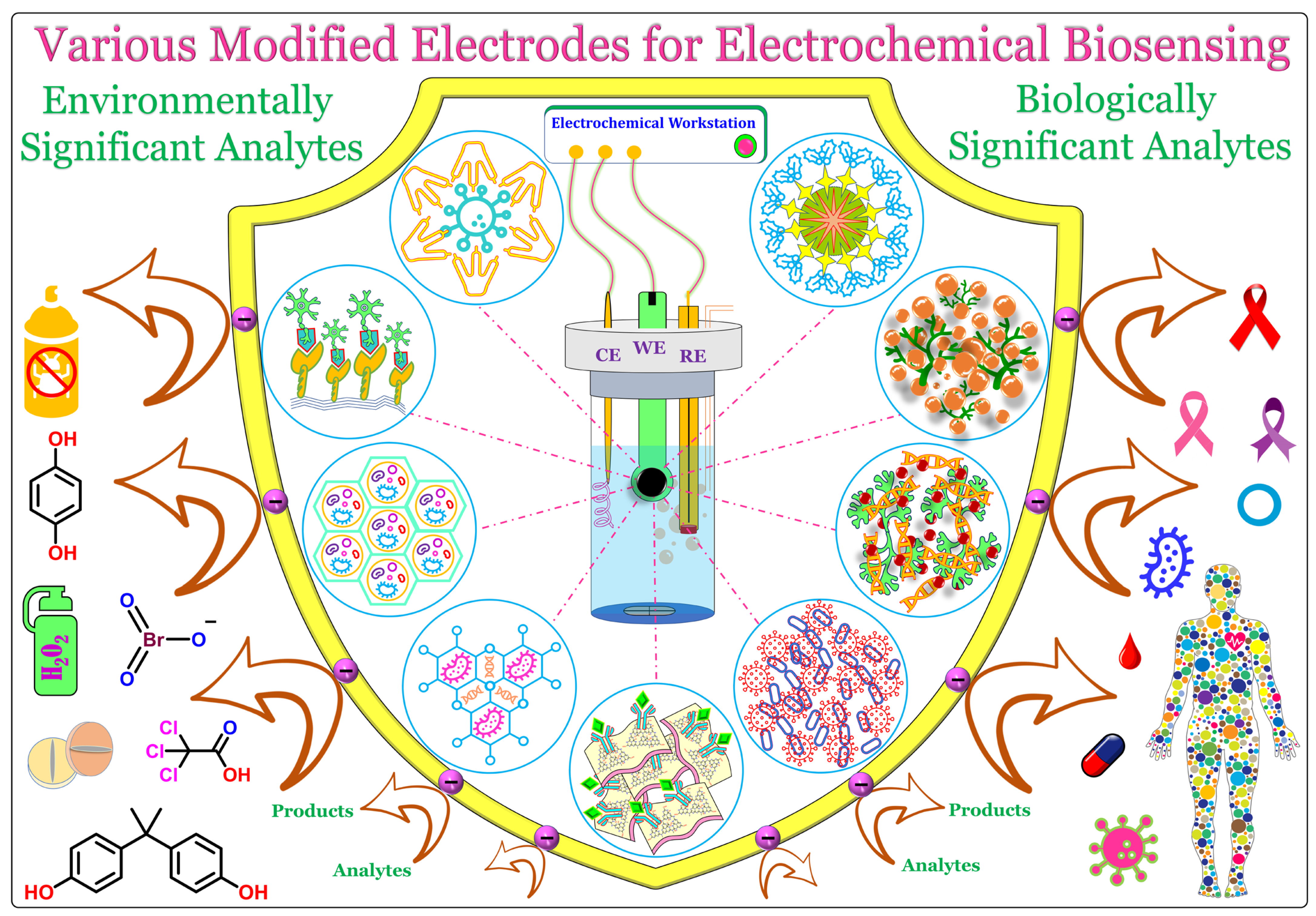
2.1. Carbon-Based Nanomaterials
2.1.1. Carbon Black
2.1.2. Graphene and Its Derivatives
Graphene (GR), the thinnest material utilized so far, is made of a single layer of carbon atoms arranged in a 2D honeycomb lattice structure with sp2 hybridization. GR is an exfoliated monolayer of graphite and has only a 1-atom thickness [46]. GR is an electrocatalytically active and transparent material with various advantages, such as high mechanical strength, excellent conductivity due to extended π conjugations, high thermal and chemical stability, large surface area, exceptional biocompatibility, and so forth. However, isolating a single layer of GR is highly complicated, as the layers are often agglomerate with one another. Due to its significant advantages in various fields, researchers have developed many derivatives of GR; some of them are few-layered GR (FLGR); GR nanosheets (GRNS); GR nanoribbons (GRNR); graphene oxide (GO); and thermally, chemically, and electrochemically reduced GO derivatives (TRGO/CRGO/ERGO) [47][48]. Among them, GO and RGO are mainly studied due to their unique physical and chemical properties, stability, and ease of synthesis. In electrochemical sensors, GO and RGO are the most preferred electrode materials and modifiers due to their excellent conductivity, electrochemical stability, exceptional support behavior, hydrophilicity, biocompatibility, rigidity, etc. Poletti and colleagues developed a novel platform for the sensing of glucose and lactate in sweat using a PB- and CS-functionalized GO-based sensor [49]. GOD and lactate oxidase (LOx) were immobilized over the developed PB/CS-GO platform. This developed sensor showed excellent electrocatalytic activity towards glucose and lactate over an upper limit linearity of 3.8 mM and 50 mM, respectively, with a limit of quantifications (LOQ) of 32 nM and 68 nM, respectively. Subsequently, an electrochemical device was constructed for the simultaneous determination of glucose and lactate in body sweat. In another study, a nanocomposite made of GO-covered GRQDs was developed for the immobilization of dsDNA and employed for the sensing of dimethoate (DMT) [50]. Initially, GRQDs were electrodeposited over GCE, and then GO was drop cased to obtain the GO@GRQDs nanocomposite. Later, dsDNA was coated over the modified electrode by applying a constant potential to form the desired biosensor. These fabricated biosensors showed excellent electrocatalytic activity towards DMT over the concentration range of 1 fM to 0.1 nM with a LOD of 1 fM. Furthermore, the sensors showed excellent selectivity, good sensitivity, rapid response, and acceptable reproducibility. Saleem and Guler developed an electrochemical biosensor for the detection of paracetamol using PdNPs-decorated CGO-modified GCE [51]. Erden and colleagues developed a novel platform using GO and polyvinyl ferrocene (PVFc) modified SPCE for the immobilization of diamine oxidase (DAOx) and/or monoamine oxidase (MAOx), and utilized it for the biosensing of tyramine [52].2.1.3. Carbon Nanotubes
Carbon nanotubes (CNTs) are 1D cylindrical nanomaterials with a few nanometers of diameter and micrometer scale lengths. Structurally, CNTs just appear as rolled-up sheet(s) of graphene, and they are categorized into single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). CNTs have various unique physiochemical properties, such as high conductivity, large surface area, good thermal and chemical stability, high tensile strength, ease of functionalization, excellent electrochemical properties, and good biocompatibility. Due to these advantages, CNTs are continuously being used in the fabrication of electrochemical sensors and biosensors [53][54]. However, aggregation of CNTs is one of the major challenges faced by researchers, which can be avoided by incorporating various functional groups by covalent modification, by creating defects, or by physically adsorbing other nanostructures over CNTs. Moreover, modification of CNTs by the aforementioned methods improves the conductivity and stability, and imparts specificity to CNTs. An electrochemical biosensor was developed using cytochrome c (Cyt-c) immobilized over MWCNTs-modified SPCE for the biosensing of fentanyl [55]. The Cyt-c enzyme was immobilized over MWCNT/SPCE via Gld cross-coupling and the developed sensor selectively detected fentanyl at a LOD of 86 ng/mL in phosphate buffer (pH 7.5). Moreover, the developed biosensor was extended for the detection of fentanyl spiked in urine samples. In another study, a glucose biosensor was fabricated using in situ grown CNTs over a gold microelectrode printed on a glass substrate [56]. Finally, GOD was encapsulated by a polymer matrix made of paraphenylenediamine (p-PDA) over the CNT/AuE to obtain the desired biosensor. The developed sensor showed excellent electrochemical oxidation of glucose with a LOD of 0.2 µM in phosphate buffer (pH 6.5). Alvarez-Paguay and colleagues developed an H2O2 biosensor using HRP and hydroxyapatite-functionalized CNTs (HAp-CNTs) modified GCE [57]. HAp-CNT protects the bioactivity and improves the conductivity of immobilized HRP. The developed sensor exhibited a wide linear detection range with a LOD of 1.91 µM for H2O2-sensing in 0.1 M phosphate buffer (pH 7.0). Subsequently, the sensor was employed for the detection of H2O2 in real samples, such as fresh and pasteurized milk samples, which showed acceptable recoveries. In a recent attempt, Bravo and colleagues developed a BPA biosensor using MWCNT and Laccase (Lac) bioconjugate [58]. A bioconjugate consisting of Lac, CS, and BMIM TFB ionic liquid in a proportion of 5:5:2 was fabricated in order to improve the stability and electrocatalytic activity of Lac, while MWCNT was used to enhance the electrochemically active surface area and electron transferability of the developed sensor. The developed sensor exhibited a broad linear range with a LOD of 8.4 nM in 0.04 M BR buffer (pH 5.0) and was also extended for the determination of BPA in river water samples. Yang and colleagues developed a point-of-care uric acid-monitoring electrochemical biosensor using urease-modified super-aligned CNTs (SACNT) [59].2.2. Metal Nanomaterials
Metal nanomaterials (MNMs) of different shapes, sizes, and dimensions, such as nanoparticles, nanosheets, nanocomposites, nanoflakes, and nanodots are being continuously utilized and explored in the design and fabrication of electrochemical biosensors due to the various following reasons: (i) MNMs have a large surface area due to their nano size, which would facilitate better loading of biomolecules on the electrode surface; (ii) since MNMs are made of metal atoms, they have a large number of free electrons, which would facilitate the electron transferability, thereby improving the conductivity and performance of the developed sensors; (iii) many MNMs have excellent biocompatibility, which is a heavily favored factor that keeps the biomolecules active and prevents them from denaturing; (iv) by suitably tuning the shape, size, and functionality of MNMs, researchers can improve the sensitivity and selectivity of the developed sensors; (v) MNMs have excellent catalytic and electrocatalytic properties; and (vi) many MNMs can be synthesized by green methods, thereby reducing environmental hazards and decreasing the overall cost of biosensor development [60][61].2.2.1. Metal Nanoparticles
Modifying the electrode surface with metal nanoparticles (MNPs) enhances biomolecule loading and improves the electrocatalytic properties of the developed biosensors. A mycobacterium tuberculosis DNA (MTb) biosensor was developed using a simple copper NP (CuNPs) with an ssDNA-modified platinum electrode (PtE) [62]. Here, the CuNPs was electrochemically deposited (−0.39 V for 60 s at 80 °C) over a precleaned PtE using copper salt dissolved in green solvent (deep eutectic solvent: a mixture of choline chloride and urea) as the supporting electrolyte. The applied potential, time, and temperature were optimized for better performance. Subsequently, a well-aligned DNA monolayer immobilization was carried out by immersing the CuNPs/PtE in an ssDNA solution for 5 h and then in mercaptohexanol (MCH). The developed sensor showed a LOD of 1 nM with a sensitivity of 42.5 µA nM−1 cm−2. Deposition of CuNPs on PtE synergistically enhanced the electrocatalytic properties of ssDNA and a biocompatible microenvironment was provided by the CuNPs, which retained the nativity of the DNA. In another work, a label-free biosensor was developed for COVID-19 antibody tests using citrate-capped spherical GNPs modified with chemically synthesized peptide receptors [63]. The biosensor was fabricated by modifying the GCE surface with a well-dispersed solution of GNPs, and then the peptide layer containing PEP2003 (five amino acids: Asn-Asn-Ala-Thr-Asn-COOH) and S protein were dropped and incubated. Here, purposefully negatively charged GNPs were used in order to firmly adsorb the peptide on the NPs and to prevent their agglomeration, as well as to retain the dispersity of the synthesized GNPs. The developed biosensor is a label-free, fast, accurate, straightforward, and cost-effective method that can be used as a promising platform for the detection of COVID-19. The functionalization of MNPs is an emerging methodology that improves selectivity and inherits the task-specific properties from MNPs. Flavin adenine dinucleotide (FAD) functionalized GNPs were synthesized and utilized as sensing probes for the selective sensing of DA at nanomolar levels (LOD, 525 nM) [64].2.2.2. Metal Oxide and Sulfide Nanoparticles
An electrochemical biosensor was developed by immobilizing GOD on zinc oxide NPs (ZnONPs) and a soy protein film (SPF) modified electrode and utilized for glucose biosensing [65]. ZnONPs offered good conductivity, a high isoelectric point, and electrochemical activity; on the other hand, SPF was chosen as the support matrix for the covalent immobilization of GOD due to its good biocompatibility and stability. Yin and colleagues developed cobalt oxide NPs (CONPs, Co3O4) decorated N-doped electrospun CNF (carbon nanofiber) for the detection of DA secreted by PC12 cells [66]. The CNF was synthesized by electrospinning, followed by hydrothermal surface functionalization, and the CONPs were electrodeposited on the as-prepared CNF-modified electrode. The developed biosensor had many advantages, such as large surface area, excellent conductivity, good selectivity, and sensitivity towards DA, and exhibited a LOD of 9 nM in 0.1 M phosphate buffer (pH 7.4). Subsequently, it was extended for the detection of DA secreted by PC12 cells. Similarly, a non-enzymatic glucose sensor was developed using a straw sheaf-like CONPs-modified electrode, which showed a high sensitivity of 669 µA mM−1 cm2 with a LOD of 0.31 µM [67]. Centane and colleagues developed an electrochemical aptasensor by immobilizing the aptamer on cobalt phthalocyanines—cerium oxide (CeO) NP conjugate for the sensing of human epidermal growth factor receptor 2 (HER) [68]. Here, the aptamer HB5 was covalently linked to the modified electrode using DCC/NHS coupling via amide bond formation between the free amine groups of HB5 and free carboxylic acid groups of the conjugate. Recently, an ultrasensitive electrochemical aptasensor was constructed using Aβ42 capture aptamer covalently modified over vertically aligned tin disulfide (SnDS) NSs and utilized for Alzheimer’s disease diagnosis [69]. Firstly, SnDS NSs were grown on a carbon cloth (CC) substrate via chemical vapor deposition (CVD) and then functionalized with MPTMS to have terminal thiol groups. Later, the thiol-functionalized Aβ42 aptamer was covalently bonded to the modified CC via disulfide bond formation in order to obtain the desired aptasensor. The constructed sensor displayed excellent stability, good selectivity, and high catalytic activity due to the presence of a large number of edge-plane-active sites as a result of the vertical aligning of SnDS NSs. Moreover, the aptamer was extended for the detection of AβOs in blood serum samples. In another study, Li and colleagues constructed a GOD-immobilized over cobalt sulfide NPs (CSNPs) decorated MWCNT modified GCE and employed it for the realization of direct electron transfer (DET) of GOD and glucose biosensing [70]. The developed sensor had excellent conductivity, good biocompatibility, and a large surface area due to the presence of CSNPs along with MWCNT, which favored high loading of GOD and preserved its bioactivity. Subsequently, DET of GOD was successfully achieved on the newly developed platform, and the lowest detection limit was found to be 5 µM in 0.1 M phosphate buffer (pH 6.0). Moreover, the developed sensor was utilized for the determination of glucose spiked in human serum samples. Rohaizad et al. developed a niobium-doped titanium disulfide (Nb-TDS) for the immobilization of GOD and fabricated a glucose biosensor [71]. Nb-TDS was synthesized separately with different concentrations of Nb and its performances towards glucose sensing were compared. TDS (group 4) was chosen due to its high conductivity, cost-effectiveness, and good stability while being a group 5 element, Nb was doped to improve the electrochemical properties of TDS towards sensing. Among different combinations, Ti0.95Nb0.05S2 showed the best performance, detecting glucose selectively at +0.1 V with a LOD of 25.7 µM in phosphate buffer (pH 7.2) containing 2 mM FcMeOH. Using a flower-like MoS2 and SiO2 conjugated with DNA probes and electroactive tags, an aptasensor was developed for the simultaneous detection of the prostate cancer biomarkers sarcosine and prostate specific antigen (PSA) [72]. Here, the functional interface MoS2 was incorporated to improve DNA hybridization and accelerate the intermolecular accessibility, while the SiO2 nanoprobe amplified electrochemical detection. The fabricated aptasensor detected sarcosine and PSA at LODs 14.4 and 2.5 fg mL−1, respectively. In another attempt, MoS2 NSs decorated with GNPs and bovine hemoglobin (BHb) were constructed for the selective detection of the pesticide chlorpyrifos (CYF) [73].2.2.3. MXenes
MXenes are layered 2D nanomaterials like graphene, and are made of transition metal carbides, nitrides, and carbonitrides. MXenes are formed by acid or alkali etching of the A layer from their MAX phases. Similar to other NMs, MXenes also possess excellent properties, such as high electrical conductivity, good thermal and chemical stability, large surface area, and so forth. However, MXenes are a step ahead of other NMs in terms of the ease of large-scale synthesis, possessing excellent biocompatibility and presence of abundant surface functionalities as a result of etching, and these functionalities can be altered by suitably choosing the etchant and the etching process, due to which MXenes possesses excellent hydrophilicity [74][75][76]. Furthermore, these terminal functional groups can be sensibly utilized for the immobilization of biomolecules, which enhances the stability and performance of the developed biosensors. An H2O2 biosensor was developed by encapsulating hemoglobin (Hb) between the sheet-like structure of Ti3C2 MXenes (TMX) [77]. The direct electrochemistry of Hb was able to be achieved due to the excellent conductivity and good biocompatibility of the TMX, which facilitated the rapid electron transfer between the deeply buried heme center of Hb and TMX without altering the nativity of Hb, whereby an effective electrochemical biosensor was developed for H2O2. In another study, a mediator-free biosensor was developed by immobilizing the TYR enzyme over TMX for the rapid and ultrasensitive detection of phenol [78]. The proposed biosensor has advantages, such as ease of preparation, facilitation of electron transferability, cost-effectiveness, and exhibited superior stability and selectivity for the sensing of phenol. A tetrahedral DNA (tDNA) immobilized TMX was fabricated for the label-free electrochemical detection of gliotoxin [79]. By exploiting the excellent conductivity and large surface area of TMX, tDNA was stably immobilized and utilized for biosensing. The developed sensor exhibited a LOD of 5 pM over a linear detection range from 5 pM to 10 nM for gliotoxin sensing. Subsequently, it was employed for the detection of gliotoxin spiked in human serum samples.2.3. Quantum Dots
Quantum dots (QDs) are 0D nanostructured semi-conducting materials with a diameter of a few nanometers; in other words, it is merely a collection of a few atoms. QDs have unique optical and electronic properties relative to their bulk and compared to other nanomaterials. Along with the regular properties of nanoparticles, such as large surface area, good electron transferability, and ease of synthesis, QDs possess various other advantages as well, such as high photostability, low cost, excellent synthetic versatility, high quantum yields, and excellent quantum confinement effects. Moreover, the properties of QDs can be tuned by altering their size, shape, composition and structure. For instance, by controlling the size of QDs, one can make the QDs absorb or emit a specific wavelength of light. There are different kinds of QDs, such as carbon-based QDs (including GR, GO, etc.), functionalized QDs, doped QDs, metallic QDs, metal oxide QDs, core-shell QDs, and so forth [80][81][82]. An electrochemical biosensor for epinephrine (EP) was developed using graphene QDs (GRQDs) covalently immobilized with Lac via Gld cross-coupling [83]. A thin layer of GRQDs was formed on the clean surface of GCE, which facilitated the immobilization of Lac due to its large surface area and monodispersed nature. The developed sensor exhibited excellent electrocatalytic oxidation of EP in a wide linear concentration range with a LOD of 83 nM in phosphate–citrate buffer (pH 5.2). However, the developed biosensor showed a feeble interference for ascorbic acid (12%), uric acid (2%), and cysteine (3%) during amperometry measurement. Subsequently, real sample analysis was performed with a pharmacological product Adrenalina WZF samples. An electrochemical biosensor for H2O2, released from CA-125, was developed using antimonene QDs (AMQDs) immobilized with catalase (Catl) enzyme [84]. AMQDs were chosen due to their large surface area, good anticancer properties, and excellent biocompatibility, which facilitated Catl immobilization and H2O2 detection in 0.1 M phosphate buffer (pH 7.5). In another study, a colloidal PbS QDs and gold nanosphere (GNSp) modified electrode was developed for the immobilization of GOD and a glucose biosensor was fabricated [85]. Here, PbS QDs were used due to their large surface area, higher number of active sites, and quantum confinement, while GNSp offered excellent conductivity and good biocompatibility toward biomolecules. A DNA biosensor was constructed for the gender determination of Arowana fish using mercaptopropionic acid (MPA) capped ZnS QDs (MZSQ), GNPs, and cysteamine (cys) [86]. The bare SPE was coated with GNPs, and then cys was allowed to form a thiol linking with GNPs. The coupling of the amine ends of cys with the carboxylic acid groups of MZSQ was performed via EDC/NHS coupling. Later, the other ends of activated carboxylic groups of MZSQ were allowed to bond with amine-terminated probe DNA. The developed DNA biosensor showed an excellent electrochemical response when binding with target DNA. In another attempt, L-cysteine-functionalized ZnS QDs were developed for the fM detection of miR-200a, an ovarian cancer biomarker [87]. Richard and colleagues developed a label-free electrochemical DNA biosensor using a nanocomposite made of SnO2 QDs and GNPs for the detection of lung cancer biomarkers [88].2.4. Organic Frameworks
Organic frameworks (OFWs) are a group of compounds that have one-, two-, or three-dimensional ordered crystalline porous structures and are formed by many repeating units joined together to obtain a cage- or frame-like structure. If the framework structure contains metal (mono or multi) clusters/ions linked together with organic (bi- or multifunctional) linkers, then they are called metal organic frameworks (MOFs). On the other hand, if the repeating units are made of lighter elements such as H, B, C, N, O, etc., then they are known as covalent organic frameworks (COFs). In general, MOFs and COFs have many interesting properties, such as large surface area, tunable porosity, moderate to good conductivity, excellent synthetic versatility, and good biocompatibility. Moreover, these properties can be tailor-made to meet the requirements of specific applications.2.4.1. Metal Organic Frameworks
MOFs are not new to the field of material sciences; they have been utilized from 1995 onwards. Over the past decade, they have seen a boom, and so far, over 100,000 MOFs have been synthesized, and millions of possible MOF structures are being predicted in silico. The metal clusters are often called nodes, and the functional ligands are known as linkers or spacers [89][90][91]. An electrochemical biosensor for the detection of the pesticide TCA and the food preservative sodium nitrite was developed using GNPs electrodeposited on a magnesium MOF immobilized with Mb [92]. Here, the Mg-MOF was chosen due to its large surface area, high stability, and excellent porosity, which aid in the immobilization and stabilization of Mb. Furthermore, GNPs were incorporated on the Mg-MOF surface via electrodeposition in order to enhance the conductivity and electrocatalytic activity of the fabricated biosensor. The developed sensor electrochemically reduced TCA and nitrite at the reduction potentials of −0.298 V and −0.535 V (vs. Ag/AgCl), respectively, in phosphate buffer (pH 2.0). However, the proposed biosensor showed higher LOD values for TCA than many reported sensors. Subsequently, the developed sensor was employed for the detection of TCA in medical skin lotion samples, which showed recovery values between 102.6% and 104.7%. Similarly, GNPs electrodeposited on a MoS2-supported Cu-MOF were developed and utilized for the immobilization of the CA125 antibody and a biosensor for the detection of the ovarian cancer biomarker CA125 was fabricated [93]. MoS2 was supported to enhance the electron and ion transferability of Cu-MOFs, and GNPs were incorporated in order to improve the conductivity between the antibody and Cu-MOF. The fabricated sensor demonstrated excellent electrocatalytic activity and good selectivity towards CA125 detection over a concentration range from 0.5 mU mL−1 to 500 U mL−1. In another study, GNPs-decorated Cu-MOF was developed for the immobilization of a capture probe based on CHA-HCR (hybridization chain reaction) and an enzyme-free biosensor for the detection of MiR was fabricated [94]. Functionalization of MOFs was carried out in order to gain more control over the structure, reactivity, and specificity of the developed MOFs. A folic acid (FA) functionalized Zr-MOF (UiO-66) was developed for the enzyme-free electrochemical detection of cancer cells [95]. Zr-MOF provided a large surface area and good surface roughness; additionally, FA has a good affinity to folate receptors (FR) and easily binds with them. Hence, the developed FA-Zr-MOF was successfully applied for the detection of FR-rich cancer cells and showed the lowest detection limit of 90 cells mL−1. In another study, a nucleic acid (NA) functionalized UiO-66 was developed and utilized for the simultaneous determination of tumor biomarkers [96]. By exploiting porosity, the NA-MOF was utilized as a nanocontainer for loading redox dyes, and closed by the gatekeeper dsDNA. As a result of recognition and hybridization, the dsDNA separates from the MOF, and the electroactive redox dyes are released, which produces a measurable signal. The proposed sensor identified the tumor biomarkers MiR and let-7a simultaneously at LODs 8.2 and 3.6 fM, respectively. Subsequently, the sensor was employed for the detection of MiR and let-7a spiked in human serum and serum samples obtained from breast cancer patients, which showed acceptable recoveries. A PdNPs-embedded amine-functionalized Cr-MOF (MIL101) was synthesized and utilized for telomerase activity detection [97]. Similarly, GNPs-decorated Ce-MOF were developed for the ratiometric detection of telomerase activity [98]. Huang et al. developed an amine functionalized Co-MOF decorated with GNPs for the immobilization of Cyt-c-modified MWCNT and fabricated a nitrite biosensor [99].2.4.2. Covalent Organic Frameworks
COFs are porous crystalline organic polymers composed of building blocks of monomer precursors. In other words, a COF is a repeating combination of two or more covalently connected organic molecules in a 2- or 3-dimensional framework. The degree and the intrinsic order are pre-determinable, and they can be rationally designed by suitably choosing the monomer with the desired functionality at the desired position. COFs have superior properties, such as large surface area, good thermal stability, low density, excellent porosity with tunable pore size, synthetic versatility, and so forth [100][101][102][103]. A triazine-based 2D COF (TCOF) was developed for the immobilization of superoxide dismutase (SOD) and an electrochemical biosensor for the biosensing of superoxide anions was fabricated [104]. The biosensor was developed by drop casting the composite (optimized quantities of TCOF, SOD, Gld, and gelatin polymer) over a precleaned SPE. TCOF improved the loading, stability, and activity of SOD due to its excellent porosity, large surface area, good conductivity, and biocompatibility. The developed COF-based biosensor showed a linear detection range from 10 nM to 100 µM with a LOD of 0.5 nM for the detection of superoxide anions. However, to reach the steady state current, the developed sensor took approximately 30 s during the amperometry measurements. Subsequently, the sensor was tested for its clinical applicability by analyzing healthy and cancerous tissue samples, which showed a good response. Xiao and colleagues developed a biosensor for carbaryl detection using nitrogen- and oxygen-rich COFs modified with ACLE [105]. The free terminal groups such as -NH, -OH and C=O of COFs were utilized for the covalent coupling of ACLE within the pores of COFs, thereby stabilizing and improving the electrochemical activity of ACLE. During electrocatalysis, ACLE turns to thiocholine (TCL), which attracts the ferri- and ferrocyanide anions in the electrolyte solution, thereby showing a well-defined redox signal, whereas in the presence of carbaryl, TCL formation was inhibited and hence a poor redox signal was observed. Using this phenomenon, a paper-based turn-off electrochemical biosensor was constructed, which showed a linear detection range from 0.48 to 35 µM with a LOD of 0.16 µM in 0.1 M KCl with 5 mM of potassium ferri- and ferrocyanide as a redox probe. In another attempt, HRP was covalently immobilized over a magnetic COF and an electrochemical biosensor for the determination of hydroquinone (HQ) was developed [106]. The magnetic COF was synthesized in situ by adding Fe3O4 during COF formation. Thereafter, HRP and Gld were added to the magnetic COF and allowed the HRP and COF to covalently couple. The iron oxide particles were accumulated within the pores of COFs and the HRP molecules covalently bonded to the terminal functional groups of the COF. The developed electrocatalyst was drop coated over GCE and employed for the determination of HQ, which showed a LOD of 120 nM in 0.1 M phosphate buffer. Subsequently, it was employed for the determination of HQ spiked in tap, lake, and wastewater samples, which showed recovery ranges between 98.4% and 104.8%. Similarly, GNPs-deposited magnetic COF was developed for the immobilization of DNA substrate and utilized for the detection of adenosine triphosphate (ATP) [107]. The GNPs were well confined in the uniform nanoporous structure of magnetic COFs, which prevented them from agglomeration. Furthermore, the presence of GNPs improves the electronic conductivity and electrocatalytic activity of the developed electrocatalyst. The developed electrocatalyst successfully reduces 4-nitrophenol (4-NP) under normal circumstances. However, in the presence of ATP, the 4-NP reduction was suppressed due to the formation of dendritic DNA strands. Based on this, an ATP biosensor was developed, which showed a broad linear detection range with a LOD of 1.6 pM and was further extended for ATP detection in serum samples. Han and colleagues developed an exosome biosensor using COF conjugated with DNA and HRP [108].2.5. Ionic Liquids
Ionic liquids (ILs) are smart molecules, completely made of ions, such as cations and anions, and held together by the electrostatic force of interactions. Unlike other molecules, ILs are entirely composed of charged species nothing but positive and negative ions and thus, it possesses various unique physiochemical properties, such as high conductivity, larger electrochemical stability, good biocompatibility, excellent thermal stability, less toxicity, non-volatility, negligible vapor pressure, and excellent synthetic versatility [109][110]. Another interesting advantage is that these physiochemical properties can be altered and tuned, depending upon the intended applications, such as changing the solubility from hydrophilic to hydrophobic, tuning the viscosity from less viscous to more viscous, or changing the physical state from solid powder to liquid gel, and suitable functionality can be introduced either on the cation or anion, or even on both the ions. Since, ILs interact with biomolecules in a non-destructive way, they are favorable candidates for the immobilization and stabilization of biomolecules such as enzymes, proteins, DNA, antibodies, etc. Due to these reasons, ILs are being considered as a potential candidate for the fabrication of electrochemical sensors and biosensors [111][112]. In the beginning, ILs were only used as conducting binders in the fabrication of composite electrodes, such as CPE in electrochemical sensors. However, after the exploration of IL possibilities in electrocatalysis, researchers have started using ILs as electrode modifiers and that is how IL-modified electrodes came into the fabrication of electrochemical sensors. Furthermore, due to their excellent biocompatibility and enhanced conductivity, ILs are employed in the development of electrochemical biosensors in order to preserve nativity, as well as to improve the activity of the immobilized biomolecules [113][114].2.5.1. Functionalized ILs
A novel amine-functionalized IL (AMIL) was rationally synthesized for the covalent immobilization of Hb and employed for the biosensing of bromate [115]. Initially, the GCE was drop coated with AMIL, and then coated with terephthaloyl chloride (TPC), a bifunctional linker, in order to form a stable amide bond between the AMIL and TPC. Later, an optimized volume of Hb was drop casted over the TPC-AMIL and allowed for covalent immobilization with the other terminal of TPC to form Hb-AMIL/GCE. The direct electrochemistry of Hb was successfully achieved on the newly developed biosensor platform, which showed a wide linear detection range with a LOD of 3 µM for the detection of bromate in nitrogen-saturated 0.1 M phosphate buffer (pH 7.0). Subsequently, in order to simplify the sensor setup, an aldehyde functionalized IL (AFIL) was synthesized for the direct covalent immobilization of GOD without any usage of a bifunctional linker, and a glucose biosensor was fabricated [116]. The biosensor was constructed by drop casting the GO over a SPCE and electrochemically reducing it by potential cycling to obtain ERGO/SPCE. Later, AFIL was dropped to form AFIL/ERGO/SPCE, and then immersed in the GOD solution to covalently immobilize the GOD to AFIL via Schiff base condensation (GOD-AFIL/ERGO/SPCE). The fabricated biosensor was utilized for the electrochemical biosensing of glucose, which showed a wide linear detection range with a sensitivity and LOD of 17.7 µA/mM/cm2 and 17 µM, respectively, in 0.1 M phosphate buffer (pH 7.0).2.5.2. ILs with Nanomaterials
A molecularly imprinted polymer (MIP) biosensor was developed for BSA using CS, IL, and GR nanocomposite-modified GCE [117]. Briefly, the GCE was drop casted with IL-GR dispersion and allowed to dry under IR lamp, and then the CS solution (4% w/v was drop casted in order to obtain CS/IL-GR/GCE (mGCE). Thereafter, the electrode was subjected to electropolymerization by performing potential cycling in the presence of BSA and pyrrole to form the BSA@MIP/mGCE. Finally, the prepared electrode was immersed in 1 M H2SO4 in order to remove the BSA from the template and to form the MIP-based sensor. The developed MIP sensor exhibited a linear detection range of 0.1 ng/L to 100 µg/L with a LOD of 0.02 ng/L in phosphate buffer containing 0.1 mM of potassium ferri- and ferrocyanide (pH 7.0). Moreover, the sensor displayed excellent selectivity and stability with acceptable reproducibility, and was utilized for the detection of BSA in bovine plasma samples, which displayed recoveries from 97.0% to 101.6%. In another study, a paper-based electrochemical biosensor for glucose was developed using a screen-printed IL (MPPy TFSI) and GR electrode (SILGE) modified with Prussian blue (PB), MXene (Ti3C2Tx) nanocomposite and GOD [118]. At first, IL-GR paste was used to print the WE and CE on the paper substrate and the WE was further modified with PB-MXene composite (PB/TMX/SILGE). Finally, GOD was drop casted and covered with a layer of Nf to obtain the paper-based screen-printed biosensor, which was able to sense glucose in a wide range of concentrations with a LOD of 24.5 µM. Subsequently, the developed sensor was employed for the detection of glucose in blood plasma samples, and it exhibited an acceptable correlation with the reference method hexokinase. Moreover, the proposed method opens a new path for the design and development of paper-based printed sensors.3. Summary
Rational design of the sensor platform, followed by the sensible immobilization of biomolecules, are the crucial components in the fabrication of effectual electrochemical biosensors. The biomolecules are highly sensitive, and even a slight change in the microenvironment might result in denaturation and loss of biosensor performance [119][120]. The following are some of the key points that need to be kept in mind while designing biosensor platforms. The intended platform or the materials chosen for the fabrication of the biosensor platform should be biocompatible and nontoxic to biomolecules, since the biomolecules are made of polypeptide layers and the secondary and tertiary structures are held together by weak forces, such as hydrogen bonding, electrostatic, disulfide, and Van der Waals forces, which may unfold and disintegrate if the biocompatibility is not maintained. The second crucial factor is conductivity; the intended platform material should be highly conductive, since the active centers are deeply buried inside the thick, non-conducting polypeptide layers, and the material should be capable enough to connect to or communicate with the active centers. For instance, the FAD redox center in GOD, the iron center in Hb, and Cyt-c are all deeply buried inside the protein layers and in order to realize its activity, the platform should communicate the active centers without damaging the secondary and tertiary structures [121][122]. The third important factor is the method of coating or immobilization of the biomolecules on the modified electrode surface. Some of the commonly used strategies are physical mixing or composite formation; drop casting (adsorption); dip coating; entrapping or encapsulating within the pores, cavities, or covers with polymer membranes such as Nafion, etc.; covalent bonding, cross-coupling, SAM and so forth. If the biomolecules are physically held on the surface by adsorption or drop casting, there is a possibility that the biomolecules may leach out during potential cycling. On the other hand, entrapment or encapsulation of biomolecules within the porous network may retain the biomolecules more effectively than adsorption and physical mixing, but they may not involve actively, due to the improper orientation of biomolecules. Therefore, the orientation of the immobilized biomolecules also needs to be maintained in order to display an effective performance. Covalent binding via a bifunctional linker and SAM formation directed by specific functional groups may produce more stable and effective biosensors, since the biomolecules are coupled to the platform strongly and uniform orientations are maintained properly. Moreover, direct covalent immobilization is preferred compared to other methods, since it does not require a bifunctional linker or other external components, such as polymers or Nafion, in order to hold the biomolecules on the substrate. Furthermore, the stability of the fabricated platform plays a significant role in the performance of the developed biosensor, and its stability depends on how well the enzymes are immobilized on the biosensor platform. Therefore, a stable and effective immobilization of biomolecules improves the shelf life and performance of fabricated biosensors. Moreover, the developed platform should have a large surface area, so that more biomolecules could be accommodated on its surface. Finally, the reaction and storage conditions also matter and determine the stability and functioning of the biosensor. For instance, if the pH of the electrolyte solution is too low or too high, it might inhibit the biomolecules’ activity and result in poor performance. Similarly, if the temperature and/or the electrochemical reaction potential is too high, it would denature the biomolecule permanently and result in the failure of the biosensor. Therefore, in order to fabricate effectual biosensors, it is necessary to be prudent and pay attention to the aforementioned requisites.
References
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H.; et al. Graphene Biodevices for Early Disease Diagnosis Based on Biomarker Detection. ACS Sens. 2021, 6, 3841–3881.
- Parsa, S.F.; Vafajoo, A.; Rostami, A.; Salarian, R.; Rabiee, M.; Rabiee, N.; Rabiee, G.; Tahriri, M.; Yadegari, A.; Vashaee, D.; et al. Early Diagnosis of Disease Using Microbead Array Technology: A Review. Anal. Chim. Acta 2018, 1032, 1–17.
- Ha, M.; Lim, S.; Ko, H. Wearable and Flexible Sensors for User-Interactive Health-Monitoring Devices. J. Mater. Chem. B 2018, 6, 4043–4064.
- Pawlik, P.; Błochowiak, K. The Role of Salivary Biomarkers in the Early Diagnosis of Alzheimer’s Disease and Parkinson’s Disease. Diagnostics 2021, 11, 371.
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572.
- Lee, Y.H.; Kweon, O.Y.; Kim, H.; Yoo, J.H.; Han, S.G.; Oh, J.H. Recent Advances in Organic Sensors for Health Self-Monitoring Systems. J. Mater. Chem. C 2018, 6, 8569–8612.
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386.
- Ma, S.; Lin, M.; Tang, J.; Liu, R.; Yang, Y.; Yu, Y.; Li, G.; An, T. Occurrence and Fate of Polycyclic Aromatic Hydrocarbons from Electronic Waste Dismantling Activities: A Critical Review from Environmental Pollution to Human Health. J. Hazard. Mater. 2022, 424, 127683.
- Chormare, R.; Kumar, M.A. Environmental Health and Risk Assessment Metrics with Special Mention to Biotransfer, Bioaccumulation and Biomagnification of Environmental Pollutants. Chemosphere 2022, 302, 134836.
- Jarin, M.; Dou, Z.; Gao, H.; Chen, Y.; Xie, X. Salinity Exchange between Seawater/Brackish Water and Domestic Wastewater through Electrodialysis for Potable Water. Front. Environ. Sci. Eng. 2023, 17, 16.
- Stefan, D.S.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 14, 3939.
- Kanwal, Q.; Li, J.; Zeng, X. Mapping Recyclability of Industrial Waste for Anthropogenic Circularity: A Circular Economy Approach. ACS Sustain. Chem. Eng. 2021, 9, 11927–11936.
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536.
- Cai, Y.; Ling, Q.; Yi, Y.; Chen, Z.; Yang, H.; Hu, B.; Liang, L.; Wang, X. Application of Covalent Organic Frameworks in Environmental Pollution Management. Appl. Catal. A 2022, 643, 118733.
- Mahamood, M.; Khan, F.R.; Zahir, F.; Javed, M.; Alhewairini, S.S. Bagarius Bagarius, and Eichhornia Crassipes Are Suitable Bioindicators of Heavy Metal Pollution, Toxicity, and Risk Assessment. Sci. Rep. 2023, 13, 1824.
- Verma, N.; Velmurugan, G.V.; Winford, E.; Coburn, H.; Kotiya, D.; Leibold, N.; Radulescu, L.; Despa, S.; Chen, K.C.; van Eldik, L.J.; et al. Aβ Efflux Impairment and Inflammation Linked to Cerebrovascular Accumulation of Amyloid-Forming Amylin Secreted from Pancreas. Commun. Biol. 2023, 6, 2.
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid Detection of Apple Juice Concentrate Adulteration with Date Concentrate, Fructose and Glucose Syrup Using HPLC-RID Incorporated with Chemometric Tools. Food Chem. 2022, 370, 131015.
- Wang, X.Y.; Zhu, G.B.; Cao, W.D.; Liu, Z.J.; Pan, C.G.; Hu, W.J.; Zhao, W.Y.; Sun, J.F. A Novel Ratiometric Fluorescent Probe for the Detection of Uric Acid in Human Blood Based on H2O2-Mediated Fluorescence Quenching of Gold/Silver Nanoclusters. Talanta 2019, 191, 46–53.
- Kim, T.; Lee, J.; Lee, J.P.; Kim, B.N.; Kim, Y.H.; Lee, Y.S.; Min, J. Screening of Novel Peptides That Specifically Interact with Vitamin D Bound Biocomplex Proteins. Sci. Rep. 2023, 13, 2116.
- Celik, C.; Demir, N.Y.; Duman, M.; Ildiz, N.; Ocsoy, I. Red Cabbage Extract-Mediated Colorimetric Sensor for Swift, Sensitive and Economic Detection of Urease-Positive Bacteria by Naked Eye and Smartphone Platform. Sci. Rep. 2023, 13, 2056.
- Nukazuka, A.; Asai, S.; Hayakawa, K.; Nakagawa, K.; Kanazashi, M.; Kakizoe, H.; Hayashi, K.; Kawahara, T.; Sawada, K.; Kuno, H.; et al. Electrical Biosensing System Utilizing Ion-Producing Enzymes Conjugated with Aptamers for the Sensing of Severe Acute Respiratory Syndrome Coronavirus 2. Sens. Bio-Sens. Res. 2023, 39, 100549.
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Ţălu, Ş.; Trejo, G.; Méndez-Albores, A. Electrochemical Biosensor for Sensitive Quantification of Glyphosate in Maize Kernels. Electroanalysis 2019, 31, 927–935.
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical Performance of an Antibody-Free Assay for Plasma Aβ42/Aβ40 to Detect Early Alterations of Alzheimer’s Disease in Individuals with Subjective Cognitive Decline. Alzheimers Res. Ther. 2023, 15, 2.
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In Vivo Electrochemical Biosensors: Recent Advances in Molecular Design, Electrode Materials, and Electrochemical Devices. Anal. Chem. 2023, 95, 388–406.
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-Derivative in a Tyrosinase-Based Amperometric Biosensor for Epinephrine Detection. Electroanalysis 2021, 33, 1639–1645.
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent Advances in Hydroxyapatite-Based Electrochemical Biosensors: Applications and Future Perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542.
- Laraib, U.; Sargazi, S.; Rahdar, A.; Khatami, M.; Pandey, S. Nanotechnology-Based Approaches for Effective Detection of Tumor Markers: A Comprehensive State-of-the-Art Review. Int. J. Biol. Macromol. 2022, 195, 356–383.
- Koo, K.M.; Kim, C.D.; Ju, F.N.; Kim, H.; Kim, C.H.; Kim, T.H. Recent Advances in Electrochemical Biosensors for Monitoring Animal Cell Function and Viability. Biosensors 2022, 12, 1162.
- Li, H.; Qi, H.; Chang, J.; Gai, P.; Li, F. Recent Progress in Homogeneous Electrochemical Sensors and Their Designs and Applications. TrAC Trends Anal. Chem. 2022, 156, 116712.
- Falina, S.; Anuar, K.; Shafiee, S.A.; Juan, J.C.; Manaf, A.A.; Kawarada, H.; Syamsul, M. Two-Dimensional Non-Carbon Materials-Based Electrochemical Printed Sensors: An Updated Review. Sensors 2022, 22, 9358.
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical Sensors Based on Molecularly Imprinted Polymer on Nanostructured Carbon Materials: A Review. J. Electroanal. Chem. 2019, 840, 343–366.
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (Bio) Sensors Go Green. Biosens. Bioelectron. 2020, 163, 112270.
- Zhang, F.T.; Cai, L.Y.; Zhou, Y.L.; Zhang, X.X. Immobilization-Free DNA-Based Homogeneous Electrochemical Biosensors. TrAC Trends Anal. Chem. 2016, 85, 17–32.
- Sheikholeslam, M.; Nanda, P.; Sanati, A.; Pritzker, M.; Chen, P. Direct Electrochemistry of Hemoglobin/Peptide-Carbon Nanotube Modified Electrode for Hydrogen Peroxide Biosensing. Mater. Lett. 2023, 335, 133799.
- Lawal, A.T. Progress in Utilisation of Graphene for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 106, 149–178.
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A Novel Nitrite Biosensor Based on the Direct Electrochemistry of Hemoglobin Immobilized on MXene-Ti3C2. Sens. Actuators B 2015, 218, 60–66.
- Sha, H. Direct Electrochemistry of Hemoglobin on Electrodeposited Three-Dimensional Interconnected Graphene-Silver Nanocomposite Modified Electrode. Int. J. Electrochem. Sci. 2016, 11, 9656–9665.
- Zhang, Y.; Li, N.; Xu, Y.; Liu, X.; Ma, Y.; Huang, Z.; Luo, H.; Hou, C.; Huo, D. A Novel Electrochemical Biosensor Based on Nano Composite Material for Ultrasensitive Detection of HER2. Bioelectrochemistry 2023, 150, 108362.
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent Electrochemical Sensors and Biosensors for Toxic Agents Based on Screen-Printed Electrodes Equipped with Nanomaterials. Microchem. J. 2023, 185, 108281.
- Tseng, W.T.; Chou, Y.Y.; Wu, J.G.; Wang, Y.C.; Tseng, T.N.; Pan, S.W.; Luo, S.C.; Ho, M.L. An Electrochemical Conducting Polymer-Based Biosensor for Leukocyte Esterase and Nitrite Detection for Diagnosing Urinary Tract Infections: A Pilot Study. Microchem. J. 2023, 188, 108493.
- Ferreira, L.M.C.; Silva, P.S.; Augusto, K.K.L.; Gomes-Júnior, P.C.; Farra, S.O.D.; Silva, T.A.; Fatibello-Filho, O.; Vicentini, F.C. Using Nanostructured Carbon Black-Based Electrochemical (Bio)Sensors for Pharmaceutical and Biomedical Analyses: A Comprehensive Review. J. Pharm. Biomed. Anal. 2022, 221, 115032.
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of Carbon Black Functionalization on the Analytical Performance of a Tyrosinase Biosensor Based on Glassy Carbon Electrode Modified with Dihexadecylphosphate Film. Enzyme Microb. Technol. 2018, 116, 41–47.
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a Phage-Based Electrochemical Biosensor for Detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345.
- Melios, N.; Tsouti, V.; Chatzandroulis, S.; Tsekenis, G. Development of an All-Carbon Electrochemical Biosensor on a Flexible Substrate for the Sensitive Detection of Glucose. Eng. Proc. 2022, 16, 17.
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-Based Magneto-Immunosensor on Carbon Black Modified Screen-Printed Electrodes for Point-of-Need Detection of Aflatoxin B1 in Cereals. Anal. Chim. Acta 2022, 1221, 340118.
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond Graphene: Electrochemical Sensors and Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2017, 89, 152–166.
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene Nanomaterials: The Wondering Material from Synthesis to Applications. Sens. Int. 2022, 3, 100190.
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895.
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous Capillary-Flow Sensing of Glucose and Lactate in Sweat with an Electrochemical Sensor Based on Functionalized Graphene Oxide. Sens. Actuators B 2021, 344, 130253.
- Elshafey, R.; Abo-Sobehy, G.F.; Radi, A.E. Graphene Oxide/Graphene Quantum Dots: A Platform for Probing Ds-DNA-Dimethoate Interaction and Dimethoate Sensing. J. Electroanal. Chem. 2021, 899, 115678.
- Saleem, S.J.; Guler, M. Electroanalytical Determination of Paracetamol Using Pd Nanoparticles Deposited on Carboxylated Graphene Oxide Modified Glassy Carbon Electrode. Electroanalysis 2019, 31, 2187–2198.
- Erden, P.E.; Erdoğan, Z.Ö.; Öztürk, F.; Koçoğlu, İ.O.; Kılıç, E. Amperometric Biosensors for Tyramine Determination Based on Graphene Oxide and Polyvinylferrocene Modified Screen-Printed Electrodes. Electroanalysis 2019, 31, 2368–2378.
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486.
- Mujica, M.L.; Tamborelli, A.; Vaschetti, V.M.; Espinoza, L.C.; Bollo, S.; Dalmasso, P.R.; Rivas, G.A. Two Birds with One Stone: Integrating Exfoliation and Immunoaffinity Properties in Multi-Walled Carbon Nanotubes by Non-Covalent Functionalization with Human Immunoglobulin G. Microchim. Acta 2023, 190, 73.
- González-Hernández, J.; Moya-Alvarado, G.; Alvarado-Gámez, A.L.; Urcuyo, R.; Barquero-Quirós, M.; Arcos-Martínez, M.J. Electrochemical Biosensor for Quantitative Determination of Fentanyl Based on Immobilized Cytochrome c on Multi-Walled Carbon Nanotubes Modified Screen-Printed Carbon Electrodes. Microchim. Acta 2022, 189, 483.
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical Biosensors Based on in Situ Grown Carbon Nanotubes on Gold Microelectrode Array Fabricated on Glass Substrate for Glucose Determination. Microchim. Acta 2023, 190, 55.
- Alvarez-Paguay, J.; Fernández, L.; Bolaños-Méndez, D.; González, G.; Espinoza-Montero, P.J. Evaluation of an Electrochemical Biosensor Based on Carbon Nanotubes, Hydroxyapatite and Horseradish Peroxidase for the Detection of Hydrogen Peroxide. Sens. Bio-Sens. Res. 2022, 37, 100514.
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase Bioconjugate and Multi-Walled Carbon Nanotubes-Based Biosensor for Bisphenol A Analysis. Bioelectrochemistry 2022, 144, 108033.
- Yang, M.; Wang, H.; Liu, P.; Cheng, J. A 3D Electrochemical Biosensor Based on Super-Aligned Carbon NanoTube Array for Point-of-Care Uric Acid Monitoring. Biosens. Bioelectron. 2021, 179, 113082.
- Lu, C.; Zhou, S.; Gao, F.; Lin, J.; Liu, J.; Zheng, J. DNA-Mediated Growth of Noble Metal Nanomaterials for Biosensing Applications. TrAC Trends Anal. Chem. 2022, 148, 116533.
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131.
- Thao, D.V.P.; Thuy Ngan, D.T.; Tuan, D.V.; Lan, H.; Thi Nguyet, N.; Thu, V.V.; Hung, V.P.; Dinh Tam, P. Facile Preparation of Copper Nanoparticles in Environmentally Friendly Solvent for DNA Sensor Application. Mater. Today Commun. 2022, 33, 104161.
- Castro, A.C.H.; Bezerra, Í.R.S.; Pascon, A.M.; da Silva, G.H.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular Label-Free Electrochemical Biosensor Loading Nature-Inspired Peptide toward the Widespread Use of COVID-19 Antibody Tests. ACS Nano 2022, 16, 14239–14253.
- Medrades, J.d.P.; Maciel, C.C.; Moraes, A.d.S.; Leite, F.d.L.; Ferreira, M. Flavin Adenine Dinucleotide Functionalized Gold Nanoparticles for the Electrochemical Detection of Dopamine. Sens. Actuators Rep. 2022, 4, 100085.
- Rawat, C.S.; Bagaria, A. Fabrication and Characterization of an Electrochemical Glucose Biosensor Using Zinc Oxide Nanoparticles and Soy Protein Isolate Film Support Matrix. MRS Commun. 2022, 12, 930–936.
- Yin, Z.; Ji, Z.; Bloom, B.P.; Jayapalan, A.; Liu, M.; Zeng, X.; Waldeck, D.H.; Wei, J. Manipulating Cobalt Oxide on N-Doped Aligned Electrospun Carbon Nanofibers towards Instant Electrochemical Detection of Dopamine Secreted by Living Cells. Appl. Surf. Sci. 2022, 577, 151912.
- Hilal, M.; Xie, W.; Yang, W. Straw-Sheaf–like Co3O4 for Preparation of an Electrochemical Non-Enzymatic Glucose Sensor. Microchim. Acta 2022, 189, 364.
- Centane, S.; Mgidlana, S.; Openda, Y.; Nyokong, T. Electrochemical Detection of Human Epidermal Growth Factor Receptor 2 Using an Aptamer on Cobalt Phthalocyanines—Cerium Oxide Nanoparticle Conjugate. Bioelectrochemistry 2022, 146, 108146.
- Tri Murti, B.; Huang, Y.J.; Darumas Putri, A.; Lee, C.P.; Hsieh, C.M.; Wei, S.M.; Tsai, M.L.; Peng, C.W.; Yang, P.K. Free-Standing Vertically Aligned Tin Disulfide Nanosheets for Ultrasensitive Aptasensor Design toward Alzheimer’s Diagnosis Applications. Chem. Eng. J. 2023, 452, 139394.
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled Carbon Nanotubes Coated with Cobalt(II) Sulfide Nanoparticles for Electrochemical Sensing of Glucose via Direct Electron Transfer to Glucose Oxidase. Microchim. Acta 2020, 187, 80.
- Rohaizad, N.; Mayorga-Martinez, C.C.; Sofer, Z.; Webster, R.D.; Pumera, M. Niobium-Doped TiS2: Formation of TiS3 Nanobelts and Their Effects in Enzymatic Biosensors. Biosens. Bioelectron. 2020, 155, 112114.
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical MoS2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 197, 113797.
- Li, W.; Zhao, Z.; Yang, W.; Su, Q.; Na, C.; Zhang, X.; Zhao, R.; Song, H. Immobilization of Bovine Hemoglobin on Au Nanoparticles/MoS2 Nanosheets—Chitosan Modified Screen-Printed Electrode as Chlorpyrifos Biosensor. Enzyme Microb. Technol. 2022, 154, 109959.
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185.
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851.
- Mathew, M.; Rout, C.S. Electrochemical Biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782.
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An Organ-Like Titanium Carbide Material (MXene) with Multilayer Structure Encapsulating Hemoglobin for a Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B16–B21.
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D Transition Metal Carbide MXene as a Robust Biosensing Platform for Enzyme Immobilization and Ultrasensitive Detection of Phenol. Biosens. Bioelectron. 2018, 107, 69–75.
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A Label-Free Electrochemical Biosensor for Highly Sensitive Detection of Gliotoxin Based on DNA Nanostructure/MXene Nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531.
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828.
- Theyagarajan, K.; Aayushi, P.S.; Devadoss, A.; Thenmozhi, K.; Senthilkumar, S. Chapter 11. Quantum Dots-Based Disposable Sensors. In Disposable Electrochemical Sensors for Healthcare Monitoring: Material Properties and Design; Pandikumar, A., Devi, K.S.S., Eds.; Royal Society of Chemistry: London, UK, 2021; pp. 314–352.
- Taghavi, F.; Moeinpour, F.; Khojastehnezhad, A.; Abnous, K.; Taghdisi, S.M. Recent Applications of Quantum Dots in Optical and Electrochemical Aptasensing Detection of Lysozyme. Anal. Biochem. 2021, 630, 114334.
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene Quantum Dots-Based Electrochemical Biosensor for Catecholamine Neurotransmitters Detection. Electroanalysis 2018, 30, 1781–1790.
- Fatima, B.; Hussain, D.; Bashir, S.; Hussain, H.T.; Aslam, R.; Nawaz, R.; Rashid, H.N.; Bashir, N.; Majeed, S.; Ashiq, M.N.; et al. Catalase Immobilized Antimonene Quantum Dots Used as an Electrochemical Biosensor for Quantitative Determination of H2O2 from CA-125 Diagnosed Ovarian Cancer Samples. Mater. Sci. Eng. C 2020, 117, 111296.
- Zhao, Y.; Huang, J.; Huang, Q.; Tao, Y.; Gu, R.; Li, H.-Y.; Liu, H. Electrochemical Biosensor Employing PbS Colloidal Quantum Dots/Au Nanospheres-Modified Electrode for Ultrasensitive Glucose Detection. Nano Res. 2022.
- Safitri, E.; Heng, L.Y.; Ahmad, M.; Tan, L.L.; Nazaruddin, N.; Suhud, K.; Chiang, C.P.; Iqhrammullah, M. Electrochemical DNA Biosensor Based on Mercaptopropionic Acid-Capped ZnS Quantum Dots for Determination of the Gender of Arowana Fish. Biosensors 2022, 12, 650.
- Moazampour, M.; Zare, H.R.; Shekari, Z. Femtomolar Determination of an Ovarian Cancer Biomarker (MiR-200a) in Blood Plasma Using a Label Free Electrochemical Biosensor Based on L-Cysteine Functionalized ZnS Quantum Dots. Anal. Methods 2021, 13, 2021–2029.
- Richard, Y.A.; Lincy, S.A.; Piraman, S.; Dharuman, V. Label-Free Electrochemical Detection of Cancer Biomarkers DNA and Anti-P53 at Tin Oxide Quantum Dot-Gold-DNA Nanoparticle Modified Electrode. Bioelectrochemistry 2023, 150, 108371.
- Majumdar, S.; Moosavi, S.M.; Jablonka, K.M.; Ongari, D.; Smit, B. Diversifying Databases of Metal Organic Frameworks for High-Throughput Computational Screening. ACS Appl. Mater. Interfaces 2021, 13, 61004–61014.
- Yan, L.; Gopal, A.; Kashif, S.; Hazelton, P.; Lan, M.; Zhang, W.; Chen, X. Metal Organic Frameworks for Antibacterial Applications. Chem. Eng. J. 2022, 435, 134975.
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646.
- Luo, G. Electrochemical Myoglobin Biosensor Based on Magnesium Metal-Organic Frameworks and Gold Nanoparticles Composite Modified Electrode. Int. J. Electrochem. Sci. 2019, 14, 2405–2413.
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ni, G.; He, F.; Li, W.; Ming, D. Molybdenum Disulfide Supported on Metal-Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Appl. Bio Mater. 2021, 4, 5494–5502.
- Xue, Y.; Wang, Y.; Feng, S.; Yan, M.; Huang, J.; Yang, X. A Dual-Amplification Mode and Cu-Based Metal-Organic Frameworks Mediated Electrochemical Biosensor for Sensitive Detection of MicroRNA. Biosens. Bioelectron. 2022, 202, 113992.
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic Acid-Functionalized Zirconium Metal-Organic Frameworks Based Electrochemical Impedance Biosensor for the Cancer Cell Detection. Sens. Actuators B 2019, 301, 127073.
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework-Based Homogeneous Electrochemical Biosensor for Simultaneous Detection of Multiple Tumor Biomarkers. Anal. Chem. 2019, 91, 3604–3610.
- Wang, L.; Meng, T.; Liang, L.; Sun, J.; Wu, S.; Wang, H.; Yang, X.; Zhang, Y. Fabrication of Amine-Functionalized Metal-Organic Frameworks with Embedded Palladium Nanoparticles for Highly Sensitive Electrochemical Detection of Telomerase Activity. Sens. Actuators B 2019, 278, 133–139.
- Dong, P.; Zhu, L.; Huang, J.; Ren, J.; Lei, J. Electrocatalysis of Cerium Metal-Organic Frameworks for Ratiometric Electrochemical Detection of Telomerase Activity. Biosens. Bioelectron. 2019, 138, 111313.
- Huang, S.; Lu, M.; Wang, L. Cytochrome C-Multiwalled Carbon Nanotube and Cobalt Metal Organic Framework/Gold Nanoparticle Immobilized Electrochemical Biosensor for Nitrite Detection. RSC Adv. 2020, 11, 501–509.
- Martínez-Periñán, E.; Martínez-Fernández, M.; Segura, J.L.; Lorenzo, E. Electrochemical (Bio)Sensors Based on Covalent Organic Frameworks (COFs). Sensors 2022, 22, 4758.
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933.
- Zhao, F.; Liu, H.; Mathe, S.D.R.; Dong, A.; Zhang, J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2018, 8, 15.
- Yildirim, O.; Derkus, B. Triazine-Based 2D Covalent Organic Frameworks Improve the Electrochemical Performance of Enzymatic Biosensors. J. Mater. Sci. 2020, 55, 3034–3044.
- Xiao, Y.; Wu, N.; Wang, L.; Chen, L. A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors 2022, 12, 899.
- Sun, X.; Xie, Y.; Chu, H.; Long, M.; Zhang, M.; Wang, Y.; Hu, X. A Highly Sensitive Electrochemical Biosensor for the Detection of Hydroquinone Based on a Magnetic Covalent Organic Framework and Enzyme for Signal Amplification. New J. Chem. 2022, 46, 11902–11909.
- Li, H.; Kou, B.; Yuan, Y.; Chai, Y.; Yuan, R. Porous Fe3O4@COF-Immobilized Gold Nanoparticles with Excellent Catalytic Performance for Sensitive Electrochemical Detection of ATP. Biosens. Bioelectron. 2022, 197, 113758.
- Han, Y.; Lu, J.; Wang, M.; Sun, C.; Yang, J.; Li, G. An Electrochemical Biosensor for Exosome Detection Based on Covalent Organic Frameworks Conjugated with DNA and Horseradish Peroxidase. J. Electroanal. Chem. 2022, 920, 116576.
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Chaurasia, J.P.; Kumar Srivastava, A. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414.
- Cetinkaya, A.; Kaya, S.I.; Yence, M.; Budak, F.; Ozkan, S.A. Ionic Liquid-Based Materials for Electrochemical Sensor Applications in Environmental Samples. Trends Environ. Anal. Chem. 2023, 37, e00188.
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical Impedimetric Biosensors, Featuring the Use of Room Temperature Ionic Liquids (RTILs): Special Focus on Non-Faradaic Sensing. Biosens. Bioelectron. 2021, 177, 112940.
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of Ionic Liquids towards Protein Stability: A Comprehensive Overview on the Current Understanding and Their Implications. Int. J. Biol. Macromol. 2017, 96, 611–651.
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812.
- Wang, X.; Hao, J. Recent Advances in Ionic Liquid-Based Electrochemical Biosensors. Sci. Bull. 2016, 61, 1281–1295.
- Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Rationally Designed Naphthyl Substituted Amine Functionalized Ionic Liquid Platform for Covalent Immobilization and Direct Electrochemistry of Hemoglobin. Sci. Rep. 2019, 9, 10428.
- Manoj, D.; Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Aldehyde Functionalized Ionic Liquid on Electrochemically Reduced Graphene Oxide as a Versatile Platform for Covalent Immobilization of Biomolecules and Biosensing. Biosens. Bioelectron. 2018, 103, 104–112.
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly Imprinted Electrochemical Biosensor Based on Chitosan/Ionic Liquid-Graphene Composites Modified Electrode for Determination of Bovine Serum Albumin. Sens. Actuators B 2016, 225, 305–311.
- Niamsi, W.; Larpant, N.; Kalambate, P.K.; Primpray, V.; Karuwan, C.; Rodthongkum, N.; Laiwattanapaisal, W. Paper-Based Screen-Printed Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites Enabled Electrochemical Detection for Glucose Sensing. Biosensors 2022, 12, 852.
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzyme Microb. Technol. 2022, 156, 110006.
- Bisht, N.; Dwivedi, N.; Khosla, A.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Review—Recent Advances in Polydopamine-Based Electrochemical Biosensors. J. Electrochem. Soc. 2022, 169, 107505.
- Chen, J.; Nilghaz, A.; Chen, X.; Liu, S.; Tian, J. Direct Electrochemical Detection of Glucose on PEDOT Functionalized Screen-Printed Electrodes. J. Electrochem. Soc. 2022, 169, 087502.
- Shen, Y.; Qian, X.; Mi, X.; Tu, Y. An Ultrasensitive Electrochemiluminescent Sensing Platform for Oxygen Metabolism Based on Bioactive Magnetic Beads. Bioelectrochemistry 2022, 145, 108086.
