
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ian R Dadour | -- | 4861 | 2023-06-14 02:02:29 | | | |
| 2 | Lindsay Dong | -4 word(s) | 4857 | 2023-06-15 03:53:58 | | | | |
| 3 | Ian R Dadour | + 5 word(s) | 4862 | 2023-06-19 07:42:07 | | |
Video Upload Options
Forensic entomology is a branch of forensic science that incorporates insects as a part of solving crime. Insect-based evidence recovered at a crime scene can be used to estimate the minimum postmortem interval, determine if a carcass/corpse has been relocated, and contribute to the cause and manner of death. Sampling and preservation of insect material is paramount if this evidence is presented in court. To this end, a qualified forensic entomologist should attend the crime scene, however this is not always the case and such evidence is collected and preserved by a proxy. After reading this paper and following a number of essential protocols, a proxy should be able to submit insect evidence that a forensic entomologist may be able to use and present a best estimate of the time since death.
1. Introduction
2. Forensic Entomology for Crime Scene Investigations
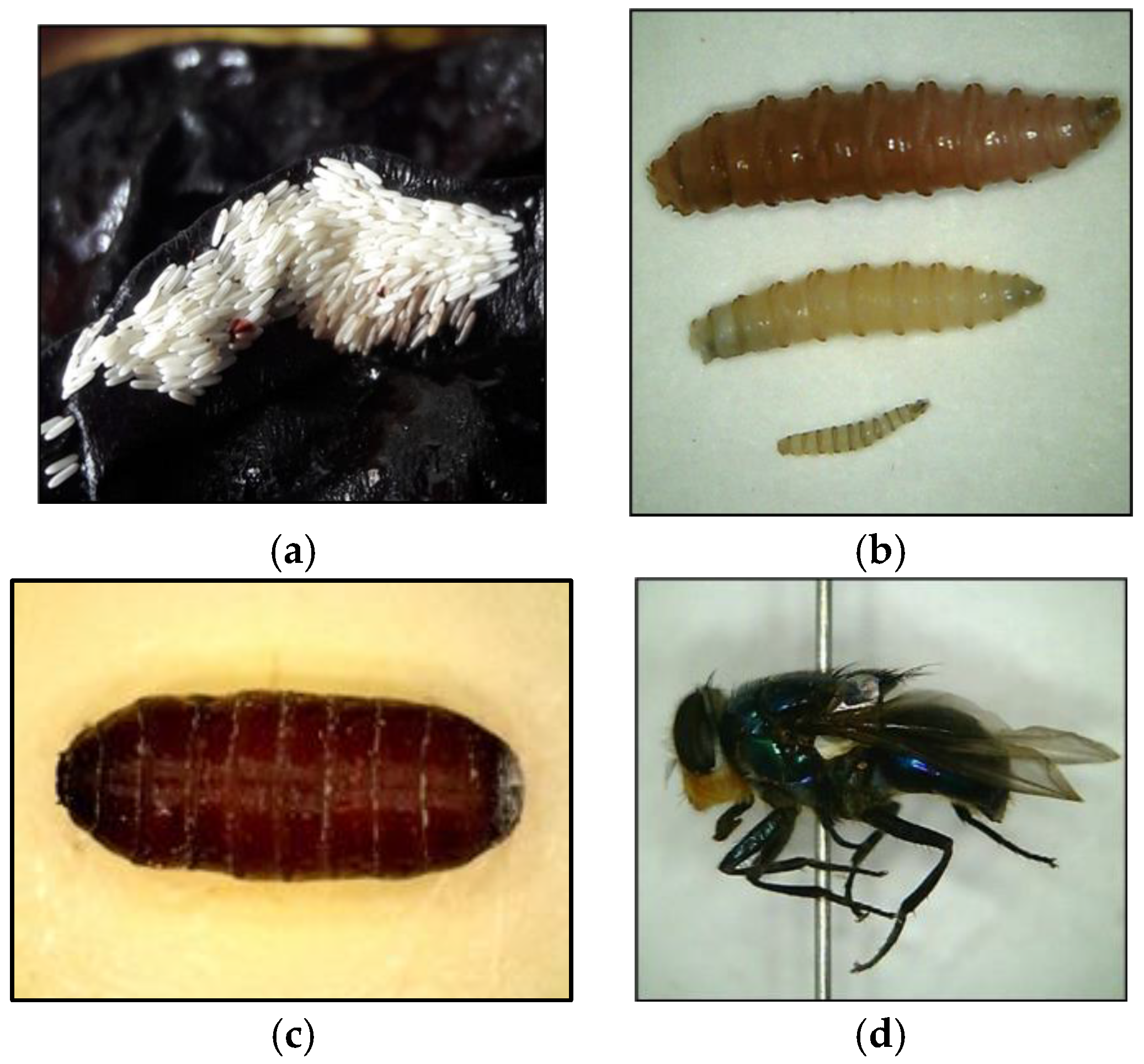
2.1. Forensic Entomologist: An Apprentice to an Expert
2.2. Forensic Entomology in Crime Investigation: Complexity and Constraints
2.3. Progression and Standardisation in Forensic Entomology
3. Crime Scene and Health Pathology Facility
3.1. Collection of Onsite Data
3.2. Collection of Microclimatic Data at a Crime Scene
3.3. Recovery of Insects for Storage or Rearing Purposes
3.3.1. Preservation of Insects
3.3.2. Preparation of Insects at the Crime Scene for Rearing Purposes
3.4. Laboratory
3.5. Identification
3.6. Examining Larvae and Full Puparia for Age Determination
3.7. Rearing
3.8. Xenobiotic Detection
4. Documentation
4.1. Referring to the Literature
4.2. Fly Development Studies
4.3. Insect Successional Studies
4.4. Casework
References
- James, S.H.; Nordby, J.J. Forensic Science: An Introduction to Scientific and Investigative Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 0-8493-1246-9.
- Saks, M.J.; Koehler, J.J. The coming paradigm shift in forensic identification science. Science 2005, 309, 892–895.
- Saferstein, R. Criminalistics: An Introduction to Forensic Science, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001.
- Brettell, T.A.; Butler, J.M.; Saferstein, R. Forensic science. Anal. Chem. 2005, 77, 3839–3860.
- Horswell, J. Crime Scene Investigation. In The Practice of Crime Scene Investigation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004.
- Katz, E.; Halámek, J. Forensic science: A multidisciplinary approach. J. Forensic Leg. Investig. Sci. 2016, 1, 1–4.
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-8153-5020-0.
- Dadour, I.R.; Morris, B. Forensic Entomology: A Synopsis, Guide, and Update. In Essentials of Autopsy Practice, 1st ed.; Rutty, G.N., Ed.; Springer: London, UK, 2014; pp. 105–130.
- Robinson, W.H. Urban Insects and Arachnids: A Handbook of Urban Entomology, 1st ed.; Cambridge University Press: Cambridge, UK, 2005; ISBN 9780521812535.
- Bugelli, V.; Tarozzic, I.; Galante, N.; Bortolini, S.; Franceschetti, L. Review on forensic importance of myiasis: Focus on medicolegal issues on post-mortem interval estimation and neglect evaluation. Legal Med. 2023, 63, 102263.
- Hagstrum, D.W.; Athanassiou, C.G. Improving stored product insect pest management: From theory to practice. Insects 2019, 10, 332.
- Haines, A.M.; Webb, S.L.; Wallace, J.R. Conservation Forensics: The Intersection of Wildlife Crime, Forensics, and Conservation. In Wildlife Biodiversity Conservation, 1st ed.; Underkoffler, S.C., Adams, H.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 125–146.
- Matuszewski, S. Post-mortem interval estimation based on insect evidence: Current challenges. Insects 2021, 12, 314.
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Rakinawasam, S.V.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B. Myiasis incidences reported in and around central province of Sri Lanka. Int. J. Dermatol. 2019, 58, 336–342.
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272.
- Forbes, S.L.; Carter, D.O. Processes and Mechanisms of Death and Decomposition of Vertebrate Carrion. In Carrion Ecology, Evolution, and Their Applications, 1st ed.; Benbow, M.E., Tomberlin, J.K., Torone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 13–30.
- Payne, J.A.; King, E.W.; Beinhart, G. Arthropod succession and decomposition of buried pigs. Nature 1968, 219, 1180–1181.
- Noriki, S.; Iino, S.; Kinoshita, K.; Fukazawa, Y.; Inai, K.; Sakai, T.; Kimura, H. Pathological analysis of cadavers for educational dissection by using postmortem imaging. Pathol. Int. 2019, 69, 580–600.
- Bajerlein, D.; Taberski, D.; Matuszewski, S. Estimation of postmortem interval (PMI) based on empty puparia of Phormia regina (Meigen) (Diptera: Calliphoridae) and third larval stage of Necrodes littoralis (L.) (Coleoptera: Silphidae)–Advantages of using different PMI indicators. J. Forensic Leg. Med. 2018, 55, 95–98.
- Pittner, S.; Bugelli, V.; Weitgasser, K.; Zissler, A.; Sanit, S.; Lutz, L.; Amendt, J. A field study to evaluate PMI estimation methods for advanced decomposition stages. Int. J. Legal Med. 2020, 134, 1361–1373.
- Bambaradeniya, Y.T.B.; Magni, P.A.; Dadour, I.R. Current Status of Five Warm Season Diptera Species in Estimating the Post-Mortem Interval. Ann. Entomol. Soc. Am. 2023, 116, 19–50.
- Kreitlow, K.L.T. Insect Succession in a Natural Environment. In Forensic Entomology, 2nd ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 251–269.
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392.
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27.
- Kulshrestha, P.; Satpathy, D.K. Use of beetles in forensic entomology. Forensic Sci. Int. 2001, 120, 15–17.
- Frost, C.L.; Braig, H.R.; Amendt, J.; Perotti, M.A. Indoor Arthropods of Forensic Importance: Insects Associated with Indoor Decomposition and Mites as Indoor Markers. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 93–108.
- Vanin, S. Advances in Forensic Entomology in Missing Persons Investigations. In Handbook of Missing Persons, 1st ed.; Morewitz, S.J., Colls, C.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 309–317.
- Prasad, S.; Aneesh, E.M. Tools and techniques in forensic entomology—A critical review. Int. J. Trop. Insect Sci. 2020, 42, 2785–2794.
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? Peer J. 2017, 5, e3506.
- Weatherbee, C.R.; Pechal, J.L.; Eric Benbow, M. The dynamic maggot mass microbiome. Ann. Entomol. Soc. Am. 2017, 110, 45–53.
- Di Luise, E.; Magni, P.; Staiti, N.; Spitaleri, S.; Romano, C. Genotyping of human nuclear DNA recovered from the gut of fly larvae. Forensic Sci. Int. 2008, 1, 591–592.
- Wells, J.D.; Stevens, J.R. Application of DNA-based methods in forensic entomology. Annu. Rev. Entomol. 2008, 53, 103–120.
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. The metagenomics worldwide research. Curr. Genet. 2017, 63, 819–829.
- Roumpeka, D.D.; Wallace, R.J.; Escalettes, F.; Fotheringham, I.; Watson, M. A review of bioinformatics tools for bioprospecting from metagenomic sequence data. Front. Genet. 2017, 8, 23.
- Carvalho, F.; Dadour, I.R.; Groth, D.M.; Harvey, M.L. Isolation and detection of ingested DNA from the immature stages of Calliphora dubia (Diptera: Calliphoridae) A forensically important blowfly. Forensic Sci. Med. Pathol. 2005, 1, 261–265.
- Baqué, M.; Amendt, J.; Verhoff, M.A.; Zehner, R. Descriptive analyses of differentially expressed genes during larval development of Calliphora vicina (Diptera: Calliphoridae). Int. J. Legal Med. 2015, 129, 891–902.
- Stewart, M.A. The teaching of entomology. J. Econ. Entomol. 1929, 22, 777–781.
- Morris, B.; Harvey, M.L.; Dadour, I.R. International Collaborations and Training. In International Dimensions & Frontiers in Forensic Entomology, 1st ed.; Tomberlin, J., Benbow, E., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Volume 30, pp. 399–416.
- Schoenly, K.G.; Haskell, N.H.; Mills, D.K.; Bieme-Ndi, C.; Larsen, K.; Lee, Y. Recreating death’s acre in the school yard: Using pig carcasses as model corpses to teach concepts of forensic entomology & ecological succession. Am. Biol. Teach. 2006, 68, 402–410.
- Magni, P.; Guercini, S.; Leighton, A.; Dadour, I. Forensic entomologists: An evaluation of their status. J. Insect Sci. 2013, 13, 78.
- Michaud, J.P.; Schoenly, K.G.; Moreau, G. Rewriting ecological succession history: Did carrion ecologists get there first? Q. Rev. Biol. 2015, 90, 45–66.
- Benecke, M.; Barksdale, L. Distinction of bloodstain patterns from fly artifacts. Forensic Sci. Int. 2003, 137, 152–159.
- Archer, M.S.; Wallman, J.F. The development of forensic entomology in Australia and New Zealand: An overview of casework practice, quality control and standards. Aust. J. Forensic Sci. 2017, 49, 125–133.
- Lutz, L.; Zehner, R.; Verhoff, M.A.; Bratzke, H.; Amendt, J. It is all about the insects: A retrospective on 20 years of forensic entomology highlights the importance of insects in legal investigations. Int. J. Legal Med. 2021, 135, 2637–2651.
- Mégnin, P. La faune des Cadavres. Application de l’entomologie a la Médicine Légal (Fauna of Cadavers. Application of Enomology in Legal Medicine); Encyclopdie scientifique des Aides-Mémoire; Les Belles Lettres: Paris, France, 1894; p. 214.
- Payne, J.A. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 1965, 46, 592–602.
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; Vanlaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421.
- Keh, B. Scope and applications of forensic entomology. Annu. Rev. Entomol. 1985, 30, 137–154.
- Tomberlin, J.K.; Byrd, J.H.; Wallace, J.R.; Benbow, M.E. Assessment of decomposition studies indicates need for standardized and repeatable research methods in forensic entomology. J. Forensic Res. 2012, 3, 1000147.
- Moreau, G. The pitfalls in the path of probabilistic inference in forensic entomology: A review. Insects 2021, 12, 240.
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M. Best practice in forensic entomology—Standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104.
- Gaudry, E.; Dourel, L. Forensic entomology: Implementing quality assurance for expertise work. Int. J. legal Med. 2013, 127, 1031–1037.
- Dadour, I.R.; Cook, D.F.; Fissioli, J.N.; Bailey, W.J. Forensic entomology: Application, education and research in Western Australia. Forensic Sci. Int. 2001, 120, 48–52.
- Hall, M.; Whitaker, A.; Richards, C. Forensic Entomology. In The Forensic Ecology Handbook: From Crime Scene to Court, 1st ed.; Marques-Grant, N., Roberts, J., Eds.; John Wiley and Sons Ltd.: Chichester, UK, 2012; pp. 111–140. ISBN 978-1-119-97419-2.
- Campobasso, C.P.; Introna, F. The forensic entomologist in the context of the forensic pathologist’s role. Forensic Sci. Int. 2001, 120, 132–139.
- Amendt, J.; Krettek, R.; Niess, C.; Zehner, R.; Bratzke, H. Forensic entomology in Germany. Forensic Sci. Int. 2000, 113, 309–314.
- Dolinski, C.; Shapiro-Ilan, D.; Lewis, E.E. Insect Cadaver Applications: Pros and Cons. In Nematode Pathogenesis of Insects and Other Pests, 1st ed.; Campos-Herrera, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 207–229.
- LeBlanc, H.N.; Logan, J.G. Exploiting Insect Olfaction in Forensic Entomology. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 205–221.
- Charabidze, D.; Depeme, A.; Devigne, C.; Hedouin, V. Do necrophagous blowflies (Diptera: Calliphoridae) lay their eggs in wounds?: Experimental data and implications for forensic entomology. Forensic Sci. Int. 2015, 253, 71–75.
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111.
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different seasons. Forensic Sci. Int. 2008, 179, 219–240.
- Bambaradeniya, Y.T.B.; Karunarathne, W.A.I.P.; Goonerathne, I.; Kotakadeniya, R.B.; Tomberlin, J.K. Use of development data to estimate colonization time of the myiasis-causing fly, Chrysomya bezziana (Diptera: Calliphoridae) collected from human wounds. Sri Lanka J. Forensic Med. Sci. Law 2016, 7, 19–26.
- Iancu, L.; Dean, D.E.; Purcarea, C. Temperature influence on prevailing necrophagous Diptera and bacterial taxa with forensic implications for postmortem interval estimation: A review. J. Med. Entomol. 2018, 55, 1369–1379.
- Guo, J.; Fu, X.; Liao, H.; Hu, Z.; Long, L.; Yan, W.; Cai, J. Potential use of bacterial community succession for estimating post-mortem interval as revealed by high-throughput sequencing. Sci. Rep. 2016, 6, 24197.
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Estimating crime scene temperatures from nearby meteorological station data. Forensic Sci. Int. 2020, 306, 110028.
- Charabidze, D.; Hedouin, V. Temperature: The weak point of forensic entomology. Int. J. Legal Med. 2019, 133, 633–639.
- Anderson, G.S.; Cervenka, V.J.; Haglund, W.; Sorg, M. Insects Associated with the Body: Their Use and Analyses. In Advances in Forensic Taphonomy: Method, Theory, and Archaeological Perspectives, 1st ed.; Haglund, W.D., Sorg, M., Eds.; CRC Press: Boca Raton, FL, USA, 2002; p. 173200. ISBN 13: 978-14200-5835-2.
- Touroo, R.; Fitch, A. Crime Scene Findings and the Identification, Collection, and Preservation of Evidence. In Veterinary Forensic Pathology, 1st ed.; Brooks, J.S., Ed.; Springer: Cham, Switzerland, 2018; pp. 9–25.
- Mohr, R.M.; Tomberlin, J.K. Development and validation of a new technique for estimating a minimum postmortem interval using adult blow fly (Diptera: Calliphoridae) carcass attendance. Int. J. Legal Med. 2015, 129, 851–859.
- Martín-Vega, D.; Hall, M.J.R. Estimating the age of Calliphora vicina eggs (Diptera: Calliphoridae): Determination of embryonic morphological landmarks and preservation of egg samples. Int. J. Legal Med. 2016, 130, 845–854.
- Hofer, I.M.; Hart, A.J.; Martín-Vega, D.; Hall, M.J. Optimising crime scene temperature collection for forensic entomology casework. Forensic Sci. Int. 2017, 270, 129–138.
- Tyndale-Biscoe, M. Age-grading methods in adult insects: A review. Bull. Entomol. Res. 1984, 74, 341–377.
- Firoozfar, F.; Moosa-Kazemi, H.; Baniardalani, M.; Abolhassani, M.; Khoobdel, M.; Rafinejd, J. Mass rearing of Lucilia sericata Meigen (Diptera: Calliphoridae). Asian Pac. J. Trop. Biomed. 2011, 1, 54–56.
- Weithmann, S.; von Hoermann, C.; Degasperi, G.; Brandt, K.; Steiger, S.; Ayasse, M. Temporal variability of the rove beetle (Coleoptera: Staphylinidae) community on small vertebrate carrion and its potential use for forensic entomology. Forensic Sci. Int. 2021, 323, 110792.
- Stamper, T.; Weidner, L.; Nigoghosian, G.; Johnson, N.; Wang, C.; Levesque-Bristol, C. Towards understanding how to instruct students in dichotomous identification keys in a mixed STEM forensic science education environment. JFSE 2020, 2, 1.
- Drijfhout, F.P. Cuticular Hydrocarbons: A New Tool in Forensic Entomology? In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009.
- Pechal, J.L.; Moore, H.; Drijfhout, F.; Benbow, M.E. Hydrocarbon profiles throughout adult Calliphoridae aging: A promising tool for forensic entomology. Forensic Sci. Int. 2014, 245, 65–71.
- Sukontason, K.; Sukontason, K.L.; Boonchu, N.; Chaiwong, T.; Piangjai, S. Ultrastructure of eggshell of Chrysomya nigripes Aubertin (Diptera: Calliphoridae). Parasitol. Res. 2004, 93, 151–154.
- Velásquez, Y.; Magaña, C.; Martínez-Sánchez, A.; Rojo, S. Diptera of forensic importance in the Iberian Peninsula: Larval identification key. Med. Vet. Entomol. 2010, 24, 293–308.
- Mendonça, P.M.; dos Santos-Mallet, J.R.; de Mello, R.P.; Gomes, L.; de Carvalho Queiroz, M.M. Identification of fly eggs using scanning electron microscopy for forensic investigations. Micron 2008, 39, 802–807.
- Ubero-Pascal, N.; Arnaldos, I.; López-Esclapez, R.; García, M.D. Microscopy and forensic entomology. Microsc. Sci. Technol. Appl. Educ. Microsc. Book Ser. 2010, 4, 1548–1556.
- Grzywacz, A. Third instar larva morphology of Hydrotaea cyrtoneurina (Zetterstedt, 1845) (Diptera: Muscidae)-a species of forensic interest. Pol. J. Entomol. 2013, 82, 303–315.
- Brunke, A.; Newton, A.; Klimaszewski, J.; Majka, C.; Marshall, S. Staphylinidae of eastern Canada and adjacent United States. Key to subfamilies: Staphylininae: Tribes and subtribes, and species of Staphylinina. Can. J. Arthropod Identif. 2011, 12, 1–110.
- Zumpt, F. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists; Butterworth & Co., Ltd.: London, UK, 1965.
- Day, D.M.; Wallman, J.F. Width as an alternative measurement to length for post-mortem interval estimations using Calliphora augur (Diptera: Calliphoridae) larvae. Forensic Sci. Int. 2006, 159, 158–167.
- Bambaradeniya, Y.T.B.; Karunaratne, W.I.P.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B.; Magni, P.A. Effect of temperature and tissue type on the development of the forensic fly Chrysomya megacephala (Diptera: Calliphoridae). J. Med. Entomol. 2019, 56, 1571–1581.
- Grassberger, M.; Reiter, C. Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen-and isomorphen-diagram. Forensic Sci. Int. 2001, 120, 32–36.
- Bambaradeniya, Y.T.B.; Karunaratne, W.I.P.; Tomberlin, J.K.; Goonerathne, I.; Kotakadeniya, R.B. Temperature and tissue type impact development of Lucilia cuprina (Diptera: Calliphoridae) in Sri Lanka. J. Med. Entomol. 2018, 55, 285–291.
- Davies, K.; Harvey, M.L. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J. Forensic Sci. 2013, 58, 79–84.
- Ma, T.; Huang, J.; Wang, J.F. Study on the pupal morphogenesis of Chrysomya rufifacies (Macquart) (Diptera: Calliphoridae) for postmortem interval estimation. Forensic Sci. Int. 2015, 253, 88–93.
- Bambaradeniya, Y.T.B.; Karunaratne, W.A.I.P.; Tomberlin, J.K.; Magni, P.A. Effect of type of tissue on the development of Chrysomya rufifacies (Diptera: Calliphoridae) in Sri Lanka. J. Med. Entomol. 2021, 58, 1673–1679.
- Yanmanee, S.; Husemann, M.; Benbow, M.E.; Suwannapong, G. Larval development rates of Chrysomya rufifacies Macquart, 1842 (Diptera: Calliphoridae) within its native range in South-East Asia. Forensic Sci. Int. 2016, 266, 63–67.
- Van der Merwe, S.S. The Identification of Diptera of the Grave and Their Succession Patterns during Winter and Summer in Central South Africa, with Reference to Forensic Applications. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2016. Available online: http://hdl.handle.net/11660/4033 (accessed on 2 May 2023).
- Swiger, S.L.; Hogsette, J.A.; Butler, J.F. Laboratory colonization of the blow flies, Chrysomya megacephala (Diptera: Calliphoridae) and Chrysomya rufifacies (Diptera: Calliphoridae). J. Econ. Entomol. 2014, 107, 1780–1784.
- Kintz, P.; Tracqui, A.; Ludes, B.; Waller, J.; Boukhabza, A.; Mangin, P.; Chaumont, A.J. Fly larvae and their relevance in forensic toxicology. Am. J. Forensic Med. Pathol. 1990, 11, 63–65.
- Pounder, D.J. Forensic entomo-toxicology. J. Forensic Sci. Soc. 1991, 31, 469–472.
- Gosselin, M.; Wille, S.M.; Fernandez, M.D.M.R.; Di Fazio, V.; Samyn, N.; De Boeck, G.; Bourel, B. Entomotoxicology, experimental set-up and interpretation for forensic toxicologists. Forensic Sci. Int. 2011, 208, 1–9.
- Introna, F.; Campobasso, C.P.; Goff, M.L. Entomotoxicology. Forensic Sci. Int. 2001, 120, 42–47.
- Hall, M.J.R. The relationship between research and casework in forensic entomology. Insects 2021, 12, 174.
- Krinsky, W.L. Forensic Entomology. Med. Vet. Entomol. 2019, 5, 51–60.
- Weidner, L.M.; Meeds, A.W.; Noblesse, A.P.; Hans, K.R. A review of Forensic Entomology literature in the southwestern United States. Wiley Interdiscip. Rev. Forensic Sci. 2021, 3, e1421.
- Matuszewski, S.; Hall, M.J.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Legal Med. 2020, 134, 793–810.





