Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abdulrahman Ismaiel | -- | 1319 | 2023-06-13 18:18:14 | | | |
| 2 | Jason Zhu | -1 word(s) | 1318 | 2023-06-14 03:43:56 | | | | |
| 3 | Jason Zhu | Meta information modification | 1318 | 2023-07-07 10:27:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sabo, C.M.; Ismaiel, M.; Ismaiel, A.; Leucuta, D.; Popa, S.; Grad, S.; Dumitrascu, D.L. Colonic Mucosal TNF-α Levels in Diverticular Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/45524 (accessed on 08 February 2026).
Sabo CM, Ismaiel M, Ismaiel A, Leucuta D, Popa S, Grad S, et al. Colonic Mucosal TNF-α Levels in Diverticular Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/45524. Accessed February 08, 2026.
Sabo, Cristina Maria, Mohamed Ismaiel, Abdulrahman Ismaiel, Daniel-Corneliu Leucuta, Stefan-Lucian Popa, Simona Grad, Dan L. Dumitrascu. "Colonic Mucosal TNF-α Levels in Diverticular Disease" Encyclopedia, https://encyclopedia.pub/entry/45524 (accessed February 08, 2026).
Sabo, C.M., Ismaiel, M., Ismaiel, A., Leucuta, D., Popa, S., Grad, S., & Dumitrascu, D.L. (2023, June 13). Colonic Mucosal TNF-α Levels in Diverticular Disease. In Encyclopedia. https://encyclopedia.pub/entry/45524
Sabo, Cristina Maria, et al. "Colonic Mucosal TNF-α Levels in Diverticular Disease." Encyclopedia. Web. 13 June, 2023.
Copy Citation
Diverticular disease (DD) is the most frequent condition in the Western world that affects the colon. Although chronic mild inflammatory processes have recently been proposed as a central factor in DD, limited information is currently available regarding the role of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α).
diverticular disease (DD)
tumor necrosis factor-alpha (TNF-α)
inflammatory markers
biomarkers
1. Introduction
Diverticulosis, a prevalent colonoscopic finding, is described as the presence of sac-like colonic protrusions. It is asymptomatic in most cases; however, about one-fourth will progress to diverticular disease (DD) [1]. This could be further divided into symptomatic uncomplicated diverticular disease (SUDD) and symptomatic complicated, which includes acute diverticulitis [1]. Additionally, segmental colitis associated with diverticulosis (SCAD) has been described as a type of colonic inflammatory disease with localized and non-granulomatous features typically limited to the sigmoid colon [2].
Diverticulosis is common in both Eastern and Western countries; however, the highest incidence was reported in the United States, Australia, and Western Europe [3]. There are differences in the anatomic diverticular distribution based on geographic areas. It mainly occurs at the right colon in Asian countries and the left colon in Western countries [4]. There is a lifetime risk from 10 to 25% of developing diverticulitis in those diagnosed with diverticulosis, and it is associated with substantial healthcare burden and morbidity [5]. About 12% of diverticulitis cases are complex, involving abscess, fistula, stricture, or perforation [6].
The pathophysiology of diverticulosis is poorly understood; nevertheless, numerous modifiable and non-modifiable factors have been associated with a high risk of diverticulosis development [7]. These include age, smoking, obesity, alcohol ingestion, lack of dietary fibers, genetic and environmental factors, abnormal colonic motility, microbiota imbalance, structural colonic wall aberrations, neuro-immune dysregulation, and mucosal inflammation [8][9]. The processes implicated in the transformation to a symptomatic diverticular status from an asymptomatic one are not clearly understood [9]. The prevalence of DD increases with age and is comparable between women and men [10].
Inflammation, both acute and chronic, plays a major role in DD and diverticulitis development and recurrence [11][12]; however, its role in the pathogenesis of diverticulosis is not very well understood at the moment [13]. Tumor necrosis factor alpha (TNF-α) is a key pro-inflammatory cytokine produced by lymphocytes, macrophages, and natural killer cells [14][15]. It is involved in multiple cellular processes and plays a role in the modulation of inflammatory pathways, such as inducible nitric oxide synthase and cyclooxygenase-2 [16].
Dysregulated TNF-α signaling has been associated with multiple inflammatory and autoimmune diseases, including rheumatoid arthritis, psoriasis, and inflammatory bowel disease (IBD) [14][17]. In IBD, there is an overexpression of numerous pro-inflammatory cytokines, including TNF-α, which results in tissue damage and stimulation of the immune system (innate and adaptive), causing chronic inflammation [16]. The use of immunotherapy, such as infliximab, a TNF-α monoclonal antibody in IBD, has been associated with remission in moderate to severe IBD cases, which reveals the significance of TNF-α in bowel disease pathology [18].
2. TNF-α Levels in SUDD Patients vs. Healthy Controls
Two studies reported the TNF-α levels in SUDD patients and control subjects [17][19]. Figure 1 outlines the obtained meta-analysis results. The pooled analysis evaluating the TNF-α levels in patients with SUDD vs. the control subjects revealed an overall MD of 0.517 (95% CI −1.148–2.182). A substantial level of heterogeneity was reported; I2 = 70% and a p-value = 0.068.
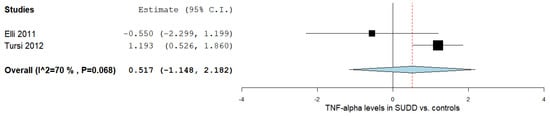
3. TNF-α Levels According to DD Status
The TNF-α levels were analyzed in a total of two studies comparing the values in symptomatic with asymptomatic DD patients, as outlined in Figure 2 [17][20]. The pooled analysis assessing the TNF-α levels in symptomatic vs. asymptomatic DD subjects revealed an overall MD of 0.657 (95% CI −0.883–2.196). A moderate level of heterogeneity was reported; I2 = 49.59% and a p-value = 0.159.
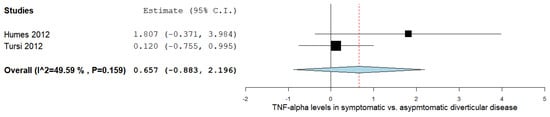
4. TNF-α Levels in DD vs. IBS
There was a significant MD between the DD and IBS subjects in the three studies comparing values on the TNF-α levels, as shown in Figure 3 [15][21][22]. The pooled analysis evaluating the TNF-α levels in DD and IBS subjects revealed an overall MD of 27.368 (95% CI 23.744–30.992). A moderate level of heterogeneity was reported; I2 = 48.75% and a p-value = 0.142.
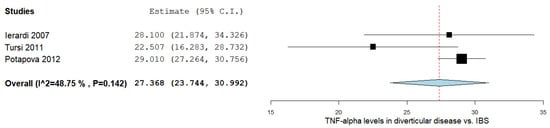
5. TNF-α Levels between SCAD and IBS Patients
In the two studies comparing the TNF-α levels in SCAD and IBS subjects [15][21], there was a significant MD between the two groups, as outlined in Figure 4. The pooled analysis of the included studies that evaluated the TNF-α levels in SCAD and IBS subjects revealed an overall MD of 25.303 (95% CI 19.823–30.784). A moderate level of heterogeneity was reported; I2 = 35.48% and a p-value = 0.213.
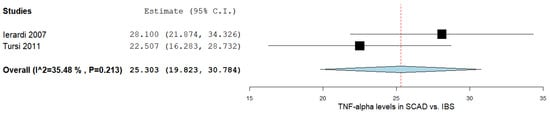
6. Qualitative Analysis
A study conducted by Ierardi et al., included 26 subjects, divided into 13 SCAD cases and 13 IBS controls [18]. Over-expression of TNF-α was observed in all SCAD patients, indicating its potential involvement in SCAD pathogenesis. Tursi et al., conducted a study in which a total of 51 participants were included, with 21 SCAD cases (8 type A SCAD, 6 type B SCAD, 3 type C SCAD, and 4 type D SCAD), and 30 serving as controls (10 IBS, 10 moderate-to-severe active UC, and 10 moderate-to-severe active ileo-colonic CD) [15]. The authors reported that TNF-α was significantly over-expressed in all SCAD patients, and higher TNF-α levels were associated with greater endoscopic damage severity. Another study conducted by Tursi et al., involved 20 subjects, including individuals with type B and type D SCAD and those with moderate-to-severe active UC [23]. The results confirmed that TNF-α expression plays a crucial role in the disease activity of SCAD, similar to its involvement in IBD. Furthermore, Tursi et al., carried out an investigation on 24 cases with DD (12 AUD, 12 SUDD) and 30 controls (12 AD, 6 SCAD, 6 UC, 6 HC), in which the authors found that TNF-α expression in DD appeared to be correlated with disease severity [17]. This was also seen in another study conducted by Tursi et al., including 22 cases with DD (15 AUD, 7 SUDD) and 37 controls (13 AD, 10 type B SCAD, 7 UC, 7 HC) [24].
Elli et al., conducted a study including 20 participants, 10 with symptomatic SUDD and 10 healthy controls [19]. The findings demonstrated the absence of inflammatory changes in the colonic mucosa of individuals with symptomatic SUDD. Moreover, Humes et al., evaluated 25 subjects, with 12 having SUDD and 13 serving as controls with asymptomatic DD [20]. The symptomatic patients exhibited a higher median relative expression of TNF alpha mRNA compared to the asymptomatic patients. Potapova et al., performed an investigation on 50 participants, including 25 with DD and 25 controls with IBS [22]. The concentration of TNF-α was significantly higher in DD compared to IBS.
Tursi et al., carried out a study involving 20 cases of ACD and 15 controls [25]. TNF-α was significantly over-expressed in ACD compared to CD, suggesting its involvement in the disease. Peery et al., performed a study on 225 individuals with DD and 364 controls [26]. No evidence was found to support the association of colonic diverticula with mucosal inflammation. Cossais et al., evaluated a total of 39 patients with DD and 23 controls [27]. Although not statistically significant, there appeared to be an increase in TNF-α expression in patients with DD compared to the controls. Moreover, Lahat et al., conducted a study that involved eight patients with ACD and eight patients with AUD [28]. The patients who experienced severe AD had higher tissue inflammatory cytokine levels compared to those with non-severe AD.
7. Bias Evaluation
The bias risk in individual studies was assessed using the NOS tool, as delineated in Supplementary materials. The NOS for cross-sectional studies was used in a total of 12 studies [15][17][18][19][20][22][23][24][25][26][27][28]. Each study had a well-defined research question and objective, while the sample size was small. Only three studies reported consecutive sampling. No study precomputed a sample size. Correcting for confounding factors, by excluding criteria in patient selection, was performed in eight studies. No matching or multiple regression techniques were employed for confounding control. All included studies correctly ascertained the exposure and the outcome. All studies used the correct statistical analysis and reported p-values. There were no problems with non-respondents in any of the studies.
References
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Prim. 2020, 6, 20.
- Freeman, H.J. Segmental colitis associated diverticulosis syndrome. World J. Gastroenterol. 2016, 22, 8067–8069.
- Violi, A.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Capasso, M.; Leandro, G.; Meschi, T.; De’Angelis, G.L.; Di Mario, F. Epidemiology and risk factors for diverticular disease. Acta Bio-Med. Atenei Parm. 2018, 89, 107–112.
- Imaeda, H.; Hibi, T. The Burden of Diverticular Disease and Its Complications: West versus East. Inflamm. Intest. Dis. 2018, 3, 61–68.
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 1282–1298.e1281.
- Yeow, M.; Syn, N.; Chong, C.S. Elective surgical versus conservative management of complicated diverticulitis: A systematic review and meta-analysis. J. Dig. Dis. 2022, 23, 91–98.
- Böhm, S.K. Risk Factors for Diverticulosis, Diverticulitis, Diverticular Perforation, and Bleeding: A Plea for More Subtle History Taking. Viszeralmedizin 2015, 31, 84–94.
- Matrana, M.R.; Margolin, D.A. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg 2009, 22, 141–146.
- Barbaro, M.R.; Cremon, C.; Fuschi, D.; Marasco, G. Pathophysiology of Diverticular Disease: From Diverticula Formation to Symptom Generation. Int. J. Mol. Sci. 2022, 23, 6698.
- Munie, S.T.; Nalamati, S.P.M. Epidemiology and Pathophysiology of Diverticular Disease. Clin. Colon Rectal Surg. 2018, 31, 209–213.
- Ceresoli, M.; Lo Bianco, G.; Gianotti, L.; Nespoli, L. Inflammation management in acute diverticulitis: Current perspectives. J. Inflamm. Res. 2018, 11, 239–246.
- Rezapour, M.; Ali, S.; Stollman, N. Diverticular Disease: An Update on Pathogenesis and Management. Gut Liver 2018, 12, 125–132.
- Tursi, A.; Elisei, W. Role of Inflammation in the Pathogenesis of Diverticular Disease. Mediat. Inflamm. 2019, 2019, 8328490.
- Jang, D.I.; Lee, A.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719.
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Ierardi, E. Tumour necrosis factor-alpha expression in segmental colitis associated with diverticulosis is related to the severity of the endoscopic damage. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2011, 43, 374–379.
- Gareb, B.; Otten, A.T.; Frijlink, H.W. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539.
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E. Musosal tumour necrosis factor α in diverticular disease of the colon is overexpressed with disease severity. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2012, 14, e258–e263.
- Ierardi, E.; Meucci, G.; Hassan, C.; Zullo, A.; Imperiali, G.; De Francesco, V.; Panella, C.; Morini, S.; Minoli, G. Tumour necrosis factor alpha in segmental colitis associated with diverticula. Dig. Dis. Sci. 2008, 53, 1865–1868.
- Elli, L.; Roncoroni, L.; Bardella, M.T.; Terrani, C.; Bonura, A.; Ciulla, M.; Marconi, S.; Piodi, L. Absence of mucosal inflammation in uncomplicated diverticular disease. Dig. Dis. Sci. 2011, 56, 2098–2103.
- Humes, D.J.; Simpson, J.; Smith, J.; Sutton, P.; Zaitoun, A.; Bush, D.; Bennett, A.; Scholefield, J.H.; Spiller, R.C. Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol. Motil. 2012, 24, 318-e163.
- Ierardi, E.; Hassan, C.; Zullo, A.; De Francesco, V.; Valle, N.D.; Prencipe, S.; Rosania, R.; Morini, S.; Panella, C. Segmental colitis associated with diverticula: A rare clinical entity and a new challenge for the gastroenterologist. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2009, 41, 794–797.
- Potapova, V.B.; Levchenko, S.V.; Gudkova, R.B.; Rogozina, V.A.; Lazebnik, L.B. Regeneration of colorectal epithelium in diverticulosis. Bull. Exp. Biol. Med. 2012, 152, 760–763.
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Ierardi, E. Tumour necrosis factor-alpha expression in segmental colitis associated with diverticulosis Down-Regulates after treatment. J. Gastrointest. Liver Dis. 2011, 20, 366–370.
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E. Mucosal expression of basic fibroblastic growth factor, Syndecan 1 and tumor necrosis factor-alpha in diverticular disease of the colon: A case-control study. Neurogastroenterol. Motil. 2012, 24, 836-e396.
- Tursi, A.; Elisei, W.; Giorgetti, G.M.; Inchingolo, C.D.; Nenna, R.; Picchio, M.; Giorgio, F.; Ierardi, E.; Brandimarte, G. Expression of basic fibroblastic growth factor, syndecan 1 and tumour necrosis factor α in resected acute colonic diverticulitis. Color. Dis. 2014, 16, O98–O103.
- Peery, A.F.; Keku, T.O.; Addamo, C.; McCoy, A.N.; Martin, C.F.; Galanko, J.A.; Sandler, R.S. Colonic Diverticula Are Not Associated With Mucosal Inflammation or Chronic Gastrointestinal Symptoms. Clin. Gastroenterol. Hepatol. 2018, 16, 884–891.e881.
- Cossais, F.; Leuschner, S.; Barrenschee, M.; Lange, C.; Ebsen, M.; Vogel, I.; Böttner, M.; Wedel, T. Persistent Increased Enteric Glial Expression of S100β is Associated with Low-grade Inflammation in Patients with Diverticular Disease. J. Clin. Gastroenterol. 2019, 53, 449–456.
- Lahat, A.; Necula, D.; Yavzori, M.; Picard, O.; Halperin, S.; Eliakim, R.; Ben-Horin, S. Prolonged Recurrent Abdominal Pain is Associated With Ongoing Underlying Mucosal Inflammation in Patients who had an Episode of Acute Complicated Diverticulitis. J. Clin. Gastroenterol. 2019, 53, e178–e185.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Entry Collection:
Gastrointestinal Disease
Revisions:
3 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




