Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dario Savoca | -- | 1630 | 2023-06-13 10:22:03 | | | |
| 2 | Camila Xu | Meta information modification | 1630 | 2023-06-13 11:18:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Physical, Chemical, and Environmental Properties of Phthalates. Encyclopedia. Available online: https://encyclopedia.pub/entry/45485 (accessed on 01 March 2026).
Savoca D, Barreca S, Lo Coco R, Punginelli D, Orecchio S, Maccotta A. Physical, Chemical, and Environmental Properties of Phthalates. Encyclopedia. Available at: https://encyclopedia.pub/entry/45485. Accessed March 01, 2026.
Savoca, Dario, Salvatore Barreca, Riccardo Lo Coco, Diletta Punginelli, Santino Orecchio, Antonella Maccotta. "Physical, Chemical, and Environmental Properties of Phthalates" Encyclopedia, https://encyclopedia.pub/entry/45485 (accessed March 01, 2026).
Savoca, D., Barreca, S., Lo Coco, R., Punginelli, D., Orecchio, S., & Maccotta, A. (2023, June 13). Physical, Chemical, and Environmental Properties of Phthalates. In Encyclopedia. https://encyclopedia.pub/entry/45485
Savoca, Dario, et al. "Physical, Chemical, and Environmental Properties of Phthalates." Encyclopedia. Web. 13 June, 2023.
Copy Citation
Phthalic acid esters (PAEs), commonly named phthalates, are a class of dialkyl or alkyl/aryl esters of phthalic acid (1,2-benzenedicarboxylic acid) structured in one benzene ring linked with two aliphatic ester groups, most commonly in the ortho configuration.
phthalic acid esters
endocrine active substances
plastic pollution
1. Introduction
Phthalic acid esters (PAEs), commonly named phthalates, are a class of dialkyl or alkyl/aryl esters of phthalic acid (1,2-benzenedicarboxylic acid) structured in one benzene ring linked with two aliphatic ester groups, most commonly in the ortho configuration [1][2]. PAEs were used for the first time as additives in plastics in the 1920s and continue to be the largest plasticiser class in the 21st century [3]. Among all the possible sources of contamination, the impact of plastics in different environmental matrices has contributed to the widespread presence of phthalates. The release of chemicals associated with plastics into the marine environment is receiving increasing attention. Phthalates are biologically active compounds that dissolve in water to varying degrees depending on the physicochemical characteristics of the side chains, particularly octanol/water partitioning (Kow). Organisms can absorb these substances by ingestion, inhalation, or contact [1].
In the organisms, PAEs are metabolised into toxic compounds that can impair vital functions. Di-2-ethylhexyl phthalate (DEHP) and di-n-butyl phthalate (DnBP) are two of the most toxic and frequently used phthalates [4].
Animal experiments have shown that phthalates interfere with normal physiological processes mediated by hormones essential for reproduction, growth, and development (e.g., decreased testis weight, spermatogenesis impairment, and external genital malformations), leading to the so-called “phthalate syndrome” [5].
Based on the concentration, the nature of the compound, the physicochemical parameters of the environment, and the organism involved, exposure to PAEs leads to different effects and levels of chronic and acute toxicity [6].
Exposure to PAEs also adversely affects the behaviour and health of adults and their offspring [7][8] causing, among others, hepatotoxicity, oxidative stress, neurodevelopmental changes, genetic aberrations, and epigenetic reprogramming [7][9][10][11][12]. Depending on effects and exposure levels, phthalates can be considered risk factors for many multifactorial diseases (e.g., reproductive pathologies, developmental alterations and embryogenesis, including the hatching success of eggs, metabolic syndromes, and tumours) [7][13]. These effects are symptomatic of a hormone balance disorder; therefore, phthalates are endocrine active substances (EAS) that can interact or interfere with normal hormonal action, showing effects of different types and severity. For this reason, they can be called modulators, perturbators, disruptors, or endocrine destroyers.
In general, EAS can act in several ways: (i) mimic the action of the hormone naturally produced, inducing an excessive response or at the wrong times (agonistic effect); (ii) block the receptor, preventing the hormone from binding there so that it cannot act (antagonistic effect); (iii) alter the regulation of hormones, acting “upstream” on their production; (iv) alter the transport of hormones in the blood [14].
PAEs are substances of concern, as reiterated in the 2021 UN report on plastic pollution [15]; consequently, restrictive measures have been introduced, limiting their use.
The regulations on the restrictions on the use of phthalates are different between international legislations; moreover, they consider only phthalates with high rates of application, and thus, high risk of exposure, which are listed as toxic, for example, di-methyl phthalate (DMP), benzyl butylphthalate (BBzP), DEHP, DnBP, di-iso-nonyl phthalate (DiNP), di-iso-decyl phthalate (DiDP), and di-n-octyl phthalate (DnOP). The restrictions mainly concern food contacts materials (FCM), cosmetics, toys, and childcare articles [2][16][17][18].
2. Physical, Chemical, and Environmental Properties of Phthalates
Phthalates are formed by a reaction of phthalic anhydride with various alcohols. The number of carbon atoms present will determine the length of the lateral chains R and R’, and thus, the molecular weight of the phthalate is obtained [1]. PAEs differ chemically in the substitutions of the R1 and R2 side chains (which characterise their physicochemical properties) and are slightly volatile liquids, generally colourless, odourless, and oily liquids at room temperature [6]. In addition, their solubility in fat (lipophilic property) increases with the lengthening of the side chains R and R’ (Figure 1).
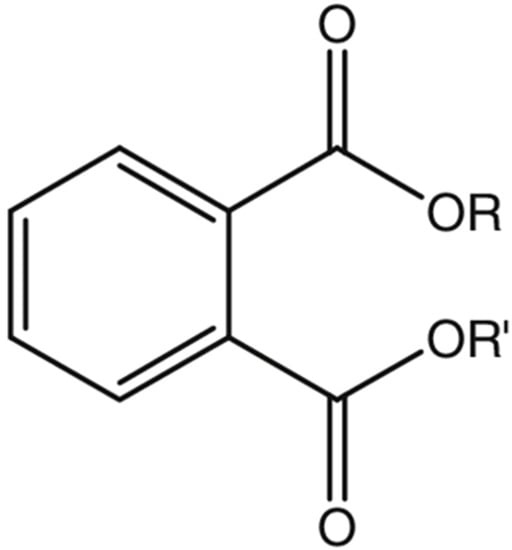
Figure 1. General chemical structure of phthalate esters.
Although there is no unique classification, it is generally possible to distinguish low molecular weight PAEs (LMW PAEs) with 3–6 carbon atoms in their side chain, and high molecular weight PAEs (HMW PAEs) with R and R′ from 7 to 13 carbons [1].
LMW PAEs include DMP, diethyl phthalate (DEP), DnBP, DiBP, and dimethylglycol phthalate (DMEP) and are typically used in PVC products, medical devices, personal care products, cosmetics, adhesives, paints, printing inks, pesticides, toys, enteric-coated tablets, food packaging or bag, etc. Most of the common phthalates are reported in Table 1.
PAEs with shorter alkyl chains, such as DMP and DEP, are widely used as solvents and fixatives, allowing fragrances, for example, to evaporate more slowly and to persist, thus extending product life [1][2][19]. HMW PAEs include BBzP, DEHP, DnOP, DiNP and DIDP, and dipropyl heptyl phthalate (DPHP), which are most commonly used as plasticisers to provide the plastic vinyl its flexibility [1].
PAEs have a relatively high boiling point and low melting point, which confers properties particularly suitable for use as plasticisers, heat transfer fluids, and carriers in the polymer industry [1]. Linear esters offer superior flexibility at low temperatures and have lower volatility than branched esters [1].
As a result of these characteristics, PAEs are widely used both as plasticisers and also as non-plasticising agents [20] in large quantities. In fact, some products may consist, by weight, of up to 40% of phthalates [21]. Despite their favourable physicochemical properties and their versatility of application in several fields that have provided numerous benefits to society, PAEs have instead demonstrated several adverse health effects of exposed organisms in all environments, especially in aquatic ones [22].
Since phthalates are not chemically bound to the polymers in which they are mixed, they can be released (for example, by contact, leaching, migration, or evaporation) in the environment, leading to exposure to the organisms present therein [22][23][24]. PAE residues have been detected in all environmental compartments. Extensive production, the storage of waste containing PAE in the environment, the inefficiency of traditional waste plants on the complete degradation, and the possible negative effects of PAEs on human health pose great global environmental and health risks for long durations [25].
Different reservoirs depend on different physicochemical properties, including water solubility (Sw), vapour pressure (Vp), Henry’s constant (KH), air/water partitioning, octanol/air partitioning (Koa), octanol/water partitioning (Kow), organic carbon partitioning (Koc), and abiotic degradation/biodegradation processes [1].
In the aquatic environment, among all the possible sources of contamination, certainly, the impact of plastics (a major source of contamination) in different environmental matrices has contributed and is contributing to their ubiquitous diffusion (due to their ability to float and resist degradation). Phthalates, favoured by the size of micro- and nano-plastics, can easily pass from low trophic levels of the food chain such as plankton and fish and then up to top predators and humans [26].
In addition, it has been shown that microplastics [27] and therefore PAEs [9] can pass through the placenta, causing exposure of the foetus to these pollutants.
PAEs’ presence has also been documented in regions far from the production areas due to the atmospheric and oceanic transport that contributes significantly to their spread [28]. This is particularly the case for short-chain phthalates, which are more susceptible to long-distance transport phenomena. As a result, they can also be found all over the world in regions where they have never been used or produced and it is very difficult to trace the source of origin [28]. To this, bioaccumulation and trophic transfer phenomena are added, further amplifying their diffusion.
The PAEs’ fate and toxicity are correlated with the wide variety of environmental and biological transformations in different compartments, which depend on the structure and the physicochemical properties of the specific PAEs, the chemical nature of the investigated matrix, as well as different environmental conditions, including organic carbon content, pH, salinity, enzyme activities, etc. [29][30][31]. PAEs, similar to other persistent organic pollutants (POPs), are subject to biomagnification phenomena with potential negative impacts on the food chain, human health, and the environment [32].
Table 1. Most common phthalates with acronyms, molecular formulas, CAS, R1, and R2 chains and their log Kow.
| PAE Congeners | Acronym | Molecular Formula | CAS | R1 | R2 | Log Kow |
|---|---|---|---|---|---|---|
| dimethyl phthalate | DMP | C10H10O4 | 131-11-3 | CH3 | CH3 | 1.60 |
| diethyl phthalate | DEP | C12H14O4 | 84-66-2 | CH2CH3 | CH2CH3 | 2.47 |
| diisobutyl phthalate | DiBP | C16H22O4 | 84-69-5 | CH2CH(CH3)2 | CH2CH(CH3)2 | 4.11 |
| dibutyl phthalate | DnBP | C16H22O4 | 84-74-2 | CH2CH2CH2CH3 | CH2CH2CH2CH3 | 4.50 |
| dimethylglycol phthalate | DMEP | C14H18O6 | 117-82-8 | CH2CH2OCH3 | CH2CH2OCH3 | 1.11 * |
| benzyl butyl phthalate | BBzP | C19H20O4 | 85-68-7 | CH2C6H5 | CH2C6H5 | 4.73 |
| dicyclohexyl phthalate | DCHP | C20H26O4 | 84-61-7 | CH(CH2)5 | CH(CH2)5 | 5.6 |
| di-n-pentyl phthalate | DnPP | C18H26O4 | 131-18-0 | CH2(CH2)3CH3 | CH2(CH2)3CH3 | 5.62 |
| bis (2-n-butoxyethyl) phthalate | DBEP | C20H30O6 | 117-83-9 | CH2CH2O(CH2)3CH3 | CH2CH2O(CH2)3CH3 | 4.06 * |
| diphenyl phthalate | DPhP | C24H38O4 | 84-62-8 | C6H5 | C6H5 | n.a. |
| di(2-ethylhexyl) phthalate | DEHP | C20H14O4 | 117-81-7 | CH(CH2)5(CH3)2 | CH(CH2)5(CH3)2 | 7.60 |
| di-n-octyl phthalate | DnOP | C24H38O4 | 117-84-0 | (CH2)7CH3 | (CH2)7CH3 | 8.10 |
| diisononyl phthalate | DiNP | C26H42O4 | 28553-12-0 | C9H19 | C9H19 | 8.8 |
| dinonyl phthalate | DnNP | C26H42O4 | 84-76-4 | C9H19 | C9H19 | 9.52 * |
Log Kow values were obtained from PubChem [33]; when the calculated value was not present, the estimated value was added *; n.a.: not available.
The danger of phthalates derives from their ability to interact with cell membranes, which is justified by their affinity towards organic portions. This property can be represented by the partition coefficient octanol/water, log Kow, i.e., the concentration ratio of a solute between octanol and water. Kow provides an estimate of the hydrophobicity of a given molecule and can predict the tendency of the breakdown of a chemical in water, lipids, sediments, and soil organic matter.
References
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655.
- Tumu, K.; Vorst, K.; Curtzwiler, G. Endocrine Modulating Chemicals in Food Packaging: A Review of Phthalates and Bisphenols. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1337–1359.
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263.
- Liu, X.; Shi, J.; Bo, T.; Li, H.; Crittenden, J.C. Occurrence and Risk Assessment of Selected Phthalates in Drinking Water from Waterworks in China. Environ. Sci. Pollut. Res. 2015, 22, 10690–10698.
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811.
- Staples, C.A.; Adams, W.J.; Parkerton, T.F.; Gorsuch, J.W.; Biddinger, G.R.; Reinert, K.H. Aquatic Toxicity of Eighteen Phthalate Esters. Environ. Toxicol. Chem. 1997, 16, 875–891.
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405.
- Montazeri, P.; Fossati, S.; Warembourg, C.; Casas, M.; Clemente, D.B.P.; Garcia-Esteban, R.; Nawrot, T.S.; Vrijheid, M. Prenatal Exposure to Phthalates and Phenols and Preclinical Vascular Health during Early Adolescence. Int. J. Hyg. Environ. Health 2022, 240, 113909.
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086.
- Qian, X.; Li, J.; Xu, S.; Wan, Y.; Li, Y.; Jiang, Y.; Zhao, H.; Zhou, Y.; Liao, J.; Liu, H.; et al. Prenatal Exposure to Phthalates and Neurocognitive Development in Children at Two Years of Age. Environ. Int. 2019, 131, 105023.
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-Ethylhexyl Phthalate: An Overview. Biomed. Res. Int. 2018, 2018, 1750368.
- Xu, S.; Zhang, H.; Pao, P.-C.; Lee, A.; Wang, J.; Suen Chan, Y.; Manno, F.A.M., III; Wan Chan, S.; Han Cheng, S.; Chen, X. Exposure to Phthalates Impaired Neurodevelopment through Estrogenic Effects and Induced DNA Damage in Neurons. Aquat. Toxicol. 2020, 222, 105469.
- Liu, Y.; Guan, Y.; Yang, Z.; Cai, Z.; Mizuno, T.; Tsuno, H.; Zhu, W.; Zhang, X. Toxicity of Seven Phthalate Esters to Embryonic Development of the Abalone Haliotis Diversicolor Supertexta. Ecotoxicology 2009, 18, 293–303.
- Ghanem, S.F. Effect of Endocrine Disrupting Chemicals Exposure on Reproduction and Endocrine Functions Using the Zebrafish Model. Egypt J. Aquat. Biol. Fish 2021, 25, 951–981.
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S.; Syed, Z.; Sonu, K.; Gupta, N.S.; Kalra, A. Perceiving Biobased Plastics as an Alternative and Innovative Solution to Combat Plastic Pollution for a Circular Economy. Sci. Total Environ. 2023, 874, 162441.
- Da Costa, J.M.; Kato, L.S.; Galvan, D.; Lelis, C.A.; Saraiva, T.; Conte-Junior, C.A. Occurrence of Phthalates in Different Food Matrices: A Systematic Review of the Main Sources of Contamination and Potential Risks. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2043–2080.
- Neves, R.A.F.; Miralha, A.; Guimarães, T.B.; Sorrentino, R.; Marques Calderari, M.R.C.; Santos, L.N. Phthalates Contamination in the Coastal and Marine Sediments of Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2023, 190, 114819.
- Carney Almroth, B.; Slunge, D. Circular Economy Could Expose Children to Hazardous Phthalates and Chlorinated Paraffins via Old Toys and Childcare Articles. J. Hazard Mater. 2022, 7, 100107.
- Orecchio, S.; Indelicato, R.; Barreca, S. Determination of Selected Phthalates by Gas Chromatography–Mass Spectrometry in Personal Perfumes. J. Toxicol. Environ. Health Part A 2015, 78, 1008–1018.
- Barreca, S.; Indelicato, R.; Orecchio, S.; Pace, A. Photodegradation of Selected Phthalates on Mural Painting Surfaces under UV Light Irradiation. Microchem. J. 2014, 114, 192–196.
- Amin, M.M.; Parastar, S.; Ebrahimpour, K.; Shoshtari-Yeganeh, B.; Hashemi, M.; Mansourian, M.; Kelishadi, R. Association of Urinary Phthalate Metabolites Concentrations with Body Mass Index and Waist Circumference. Environ. Sci. Pollut. Res. 2018, 25, 11143–11151.
- Savoca, D.; Lo Coco, R.; Melfi, R.; Pace, A. Uptake and Photoinduced Degradation of Phthalic Acid Esters (PAEs) in Ulva lactuca Highlight Its Potential Application in Environmental Bioremediation. Environ. Sci. Pollut. Res. 2022, 29, 90887–90897.
- Marturano, V.; Cerruti, P.; Ambrogi, V. Polymer Additives. Phys. Sci. Rev. 2017, 2, 20160130.
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The Polymers and Their Additives in Particulate Plastics: What Makes Them Hazardous to the Fauna? Sci. Total Environ. 2022, 824, 153828.
- Gao, D.-W.; Wen, Z.-D. Phthalate Esters in the Environment: A Critical Review of Their Occurrence, Biodegradation, and Removal during Wastewater Treatment Processes. Sci. Total Environ. 2016, 541, 986–1001.
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283.
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274.
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035.
- Ma, T.; Zhou, W.; Chen, L.; Wu, L.; Christie, P.; Liu, W. Toxicity of Phthalate Esters to Lettuce (Lactuca sativa) and the Soil Microbial Community under Different Soil Conditions. PLoS ONE 2018, 13, e0208111.
- Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Zhang, H.; Yu, M.; Ma, L.; Xi, B.; et al. Contamination of Phthalate Esters (PAEs) in Typical Wastewater-Irrigated Agricultural Soils in Hebei, North China. PLoS ONE 2015, 10, e0137998.
- Zhang, Z.-M.; Zhang, H.-H.; Zhang, J.; Wang, Q.-W.; Yang, G.-P. Occurrence, Distribution, and Ecological Risks of Phthalate Esters in the Seawater and Sediment of Changjiang River Estuary and Its Adjacent Area. Sci. Total Environ. 2018, 619–620, 93–102.
- Savoca, D.; Pace, A. Bioaccumulation, Biodistribution, Toxicology and Biomonitoring of Organofluorine Compounds in Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 6276.
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic. Acids Res. 2023, 51, D1373–D1380.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
13 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





