
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hong Duc Thi Nguyen | -- | 1407 | 2023-06-12 22:13:26 | | | |
| 2 | Dean Liu | -5 word(s) | 1402 | 2023-06-13 03:39:03 | | | | |
| 3 | Hong Duc Thi Nguyen | + 3262 word(s) | 4664 | 2023-06-18 10:57:12 | | |
Video Upload Options
Uterine cervical cancer (CC) is a complex, multistep disease primarily linked to persistent infection with high-risk human papillomavirus (HR-HPV). However, it is widely acknowledged that HR-HPV infection alone cannot account for the formation and progression of CC. Emerging evidence suggests that the cervicovaginal microbiome (CVM) also plays a significant role in HPVrelated CC. Certain bacteria, such as Fusobacterium spp., Porphyromonas, Prevotella, and Campylobacter, are currently being considered as potential microbiomarkers for HPV-positive CC. However, the composition of the CVM in CC is inconsistent; thus, further studies are needed. This review comprehensively discusses the complex interplay between HPV and the CVM in cervical carcinogenesis. It is postulated that the dynamic interaction between HPV and the CVM creates an imbalanced cervicovaginal microenvironment that triggers dysbiosis, enhances HPV persistence, and promotes cervical carcinogenesis. Moreover, this review aims to provide updated evidence on the potential role of bacteriotherapy, particularly probiotics, in the treatment of CC
1. Introduction
2. Human Papillomavirus
2.1. Structure and Genome
2.2. Life Cycle of HPV
2.3. HPV and Host Immune Responses
2.3.1. HPV and Innate Immune Response
2.3.2. HPV and the Adaptive Immune Response
2.3.3. HPV and Immune Suppression
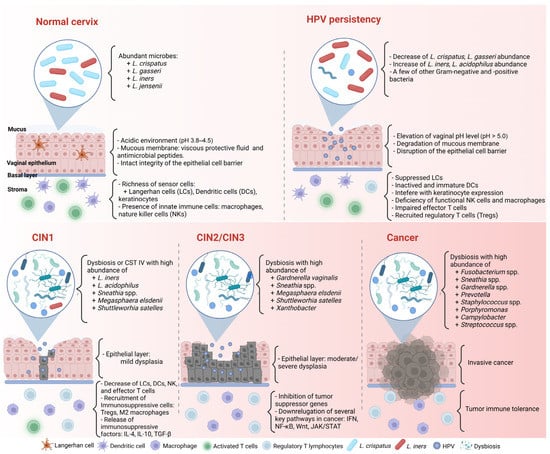
3.1. Cervicovaginal Microbiome in Healthy Women
Lactobacillus plays a critical role in cervicovaginal health in women [54]. They protect the vagina from bacterial invasion by maintaining an acidic environment and promoting the integrity of the epithelial cell barrier and intercellular junctional proteins [5,55,56].
Based on next-generation sequencing, many human microbiota studies have identified complex microbial communities in healthy women [54,57,58]. Ravel et al. [4] utilized 16S rRNA sequencing on 396 asymptomatic women of different ethnicities and investigated five community state types (CSTs). CSTs I to III and V present low diversity and are dominant with Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively. In contrast, CST IV is characterized by reduced lactobacilli abundance and high diversity with a significant quantity of vaginosis bacteria, such as Gardnerella vaginalis, Megasphaera, Sneathia, and Prevotella spp.
The CVM is dynamic; one CST can transform into another in the same healthy woman. For example, an L. crispatus-dominated community often changes to dominant L. iners or mixed lactobacilli, and the L. iners-dominated one likely transforms into a bacterial vaginosis (BV)-associated community (CST IV) [59].
The CVM composition is influenced by numerous factors, such as ethnicity, hormonal status, sexual activity, age, menstrual cycle, or menopause [5,60,61]. Women of different ethnic groups are associated with different CVMs. For example, L. iners and L. crispatus were the most abundant cervicovaginal microbial communities in healthy Asian women [62], whereas L. iners and CST IV were dominant microbial communities in Black and Hispanic women [4].
In reproductive-aged women, the cyclic secretion of hormones determines significance, especially during the menstrual phase. In Cheng et al.’s study on Chinese women’s vaginal microbiota at childbearing age [63], L. iners and Lactobacillus helveticus are the most abundant species in both follicular and luteal phases [64]. Furthermore, low estrogen and progesterone levels and existing menstrual blood in the female vagina are related to the decrease in some microorganisms and the enrichment of others. Accordingly, during menses, reproductive-aged women may have a low amount of Lactobacillus spp. and a high abundance of other species, such as G. vaginalis, Streptococcus spp., and Anaerococcus spp. [65]. The reason is that the high iron level in menstrual blood may enhance the growth of bacteria such as G. vaginalis [66].
To understand the association between the CVM and several female conditions (pregnancy history, marriage, number of vaginal deliveries, age at first instance of vaginal sex, and breastfeeding), Jie et al. [57] utilized metagenomic shotgun sequencing on cervical samples from 516 women and recorded their life histories through questionnaires. They revealed that the cervicovaginal microbiota might reflect female physical status. For instance, L. crispatus was significantly higher in women with fewer pregnancies and overrepresented in menstruation. However, its abundance was depleted during breastfeeding or postmenopause.
The CVM with predominant Lactobacillus protects the female genital tract against bacterial pathogens [5]. When lactobacilli are depleted, invading microorganisms can easily penetrate epithelial cell layers. Hence, a CVM with nondominant Lactobacillus and increased pathogenic microbial diversity, such as G. vaginalis, Atopobium, Prevotella, Sneathia, and Megasphaera, is commonly seen in BV or dysbiosis [67–71]. BV can damage the mucus and cytoskeleton structures, increase cell death, change antimicrobial peptides [62], and promote proinflammatory cytokine production [72–75].
Anahtar et al. [76] investigated a group of asymptomatic women in South Africa and found that most women had low Lactobacillus abundance and a high diversity of bacterial communities. Sneathia sanguinigens, Sneathia amnii, Mobiluncus mulieris, and Prevotellaamnii in high-diversity communities are related to the presence of genital proinflammatory cytokines, such as IL-1α, IL-1β, and IL-8. Furthermore, the bacterial lipopolysaccharide in the CVM can be sensed by antigen-presenting cells on epithelial cells, triggering NF-κB pathways and Toll-like receptors and the recruitment of lymphocytes through chemokine secretion. Laniewski et al. [77] found similar results in 3D epithelial cervical cell models co-cultured with Lactobacillus crispatus and BV-associated bacteria Gardnerella vaginalis, Atopobium vaginae, Prevotella bivia, and Sneathia amnii. Specifically, Lactobacillus crispatus enhanced protection of the cervical microenvironment through antimicrobial metabolites, including decreasing glucose and the production of phenyllactate and N-acetylated amino acids in 3D cervical models. In contrast, Atopobium vaginae and Sneathia amnii induced the greatest proinflammatory cytokines (IL-6, IL-8, TNFα, etc.), iNOS, and oxidative stress, while Gardnerella vaginalis, Prevotella bivia, and Sneathia amnii altered the epithelial barrier by decreasing protein and metabolite levels, such as mucins, sialic acid, and polyamines [77]. These alterations in the inflammatory cytokine profile present in a CVM environment may result in chronic inflammation, a risk factor for cervical carcinogenesis [73].
In addition, Fan et al. [78] reported the relationship between CVM dysbiosis and mucosal epithelial cell fucosylation, which is a protective component of vaginal epithelial cells [78]. Knocking out the core fucosyltransferase gene can promote CC cell proliferation and invasion. L. iners metabolites can increase core fucosylation levels by activating the Wnt pathway and inhibiting CC proliferation and migration. Hence, CVM imbalance and a low abundance of L. iners may result in abnormal fucosylation, which promotes the development of CC.
4. HPV Infection, Cervicovaginal Microbiome, and Cervical Carcinogenesis
The interaction between the host and microorganisms in cancer, particularly CC, is complex and poorly understood. Nevertheless, recent studies have provided critical evidence and insights into this vital field [79].
4.1. HPV Infection and Cervicovaginal Microbiome
HPV infection status is closely related to cervicovaginal dysbiosis (Figure 1) [9,70]. Di Paola et al. [80] collected cervicovaginal samples from 55 HPV-positive women in Italy to identify the CST related to persistent HPV [80]. To fulfill their purpose, they followed up with the candidates and checked the clearance or persistence of HPV after 12 months. More than 40% of persistent HPV-positive women presented in the CST IV subgroup with Gardnerella, Prevotella, Atopobium, and Megasphaera. Significantly, Gardnerella may contribute to the ongoing HPV status by secreting the sialidase enzyme involved in biofilm formation [80,81]. The association between biofilm formation in the CVM and HPV infection was confirmed by Donmez et al. [82]. In line with these results, Qingqing et al. [83] identified a high abundance of anaerobes, including Prevotella, Sphingomonas, and Anaerococcus, related to persistent HPV and a higher presence of Tregs, MDSCs, IL-6, and TNF-α in cervical secretions.
In addition, many studies have reported the association between the CVM and HRHPV infection [3,84–86]. Most revealed a decrease in Lactobacillus spp. and an increase in CVM diversity in HR-HPV-positive women than in HR-HPV-negative women. For instance, Brotman et al. [87] identified that most HR-HPV-positive groups had a four-fold lower abundance of Lactobacillus spp., including L. crispatus, L. jensenii, and L. gasseri, compared to HPV-negative groups. In particular, Lee et al. [88] compared the differences in the CVM between HR-HPV-negative and HR-HPV-positive women in a Korean twin cohort. They proved that the HPV-positive group had higher species diversity and a lower abundance of Lactobacillus than their uninfected twins. Furthermore, they suggested that Sneathnia spp. could be identified as a microbiological marker of an HR-HPV infection. Also, Lebeau et al. [9] followed up on > 6000 patients regarding their HPV infection and BV status for > 8 years. By applying multiple analyses from patients, cell line cultures, and transgenic mouse samples, they discovered that the downregulation of NF-κB and Wnt/β- catenin signaling pathways due to persistent HPV infection can lead to the inhibition of most antimicrobial peptides, such as S100A7, SLPI, Elafin, HD6, HβD2, and HD5, and impair TNF-α/LPS. Because several antimicrobial peptides are the amino acid source for Lactobacillus survival, the Lactobacillus concentration was significantly decreased. In other words, the escape of HPV from the immune response results in an imbalance of microbiota in the female vaginal flora.
HPV-persistent infection can interfere with the cervical microenvironment, cell proliferation, angiogenesis, and tissue differentiation. Any changes in regulatory factors in these processes may cause cervical neoplasia. However, HPV infection alone does not explain the development of CC. Indeed, some aspects, such as the milieu of mucosal secretion, epithelial surface integrity, immune regulation, and local microbiota, may influence HPV carcinogenesis [47]. Notably, increasing CVM diversity has been investigated to be associated with CIN progression and may be involved in regulating persistent HPV infection (Table 1) [89–91]. Guo et al. [11] identified differential microbial communities among 149 women with different HPV and SIL statuses. A non-Lactobacillus CVM was predominant in SIL women compared to HPV-negative and HPV-positive non-SILs. S. oralis and unclassified OTU265 differed between HPV-positive and HPV-positive LSILs. Also, this study revealed several unclassified OTUs, such as OTU880, OTU893, and OTU883, predominant in the HPV-positive HSIL group. In a longitudinal study, Usyk et al. [92] evaluated the CVM of 273 HR-HPV-infected women and tumor progression after two visits. They clustered the CVM into four CSTs: two CSTs related to L. iners and L. crispatus, one cluster containing high levels of G. vaginalis, and other CSTs without a significant group. L. iners were associated with HPV clearance, whereas G. vaginalis correlated with CIN2 progression after the first visit. Prevotella amnii and Anaerococcus prevotii, two common causative agents of BV, were linked to disease progression on the second visit. L. iners may have two opposite functions in the cervicovaginal microenvironment: one can promote health, and the other is associated with dysbiosis and CIN susceptibility [93]. For example, Oh et al. [94] identified a high abundance of L. crispatus in low-risk CIN, whereas L. iners was dominant in medium-risk CIN
Recently, multiomics has become an increasingly important area in cancer research. Several multiomics studies were carried out and contributed various insights into the underlying mechanism of the complex interaction between CC and its microorganisms. IIhan et al. [96] evaluated the CVM metabolomic and metagenomic profiling of 78 women in Arizona. Amino acid and nucleotide metabolism disruptions were identified in nonLactobacillus-dominant communities and in high-grade dysplasia. 3-hydroxybutyrate, eicosenoate, and oleate/vaccinate are the metabolic features of CC [96]. Another multiomics study also highlighted that pipecolate and deoxycarnitine were significantly related to vaginal dysbiosis with HPV infection. Particularly, 3-hydroxybutyrate was strongly associated with a high abundance of Streptococcus, Prevotella, Megasphaera, Atopobium, and Sneathia [97].
5. Bacteriotherapy in Cervical Cancer Treatment
5.1. Probiotic Bacteriotherapy in Cervical Cancer Treatment
In the early stages of CC, there are multiple choices for treatment, such as surgery (e.g., fertility preservation surgery), radiation, and neoadjuvant chemotherapy. In the locally advanced stage, chemoradiation is the best approach. However, 30–40% of the patients do not respond entirely, and some patients face side effects from this therapy [98]. Therefore, it is necessary to find a more effective and nontoxic or less toxic treatment [99].
Recently, bacteriotherapy has emerged as a promising platform for cancer treatment. One of the most common representatives of bacteriotherapy is probiotics. Probiotics contain a number of live microorganisms that provide several benefits to their host. There are many sources of probiotics in the human diet, such as fermented milk products (yogurt, cheese, and beer) and mainly vegetables (cabbage and cucumber) [100,101]. The most common genera of probiotics in the human diet are Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus, and Enterococcus. Also, some strains belonging to Bacillus and Saccharomyces are utilized nowadays [102].
Various studies have assessed the efficacy of probiotics on CC cell lines. For instance, on the effect of L. crispatus, L. jensenii, and L. gasseri on CaSki cells, Wang et al. [106] reported that these subgenera of Lactobacillus could inhibit CC cell proliferation through the regulation of HPV oncogenes and cell cycle-related genes. Another study showed that L. gasseri could promote apoptosis in HeLa cells through its exopolysaccharides. Also, L. gasseri could affect the anti-inflammation of HeLa cells by reducing TNF-α and increasing the cytokine IL-10 [107]. Moreover, lactobacilli and their metabolites are essential in CC prevention and treatment. Pawar et al. [108] found that cell-free culture supernatants of 12 Lactobacillus spp. from different microenvironments resembled inhibitors of HPV 16 and 18 by restoring E-cadherin and suppressing MMP9. Another strain, Bifidobacterium adolescentis SPM1005-A, has potential antiactivity by inhibiting E6 and E7 oncogene expression in SiHa cells [103].
There are also reports on the special role of probiotics in HPV infection and CC in human trials (Table 2). In 2013, a prospective controlled pilot study in women with HPV infection and LSILs who took an oral probiotic product (Lactobacillus casei) for six months had higher HPV clearance than the control group; however, the difference was not significant [14]. In a well-designed study by Out et al. [109], 121 HPV-positive women were given oral-specific strains of Lactobacillus rhamnosus and Lactobacillus reuteri daily. Although the trial effects did not influence HPV clearance, they significantly decreased the rates of mildly abnormal and unsatisfactory cervical smears. Likewise, Dellino et al. [105] found that HPV-positive women who took the long-term oral probiotic L. crispatus M247 had reduced HPV-related cytological anomalies compared to the control group. However, HPV clearance was not significantly different between the probiotic group and the group without probiotics. In contrast, Pierro et al. [15] reported an increase in HPV clearance after 90 days of taking L. crispatus M247; furthermore, in some cases, the CVM shifted the CST status to CST I.
5.2.1. Vaginal Suppositories
In addition to oral probiotics, vaginal suppositories containing Lactobacillus have been studied and developed to prevent vaginal dysbiosis, such as BV or vulvovaginal candidiasis (Table 3) [110,111]. In a clinical trial, Tomusiak et al. [111] revealed that vaginal medicinal therapy (containing Lactobacillus fermentum 57A, Lactobacillus plantarum 57B, and L. gasseri 57C) is safe and can promote an increased abundance of Lactobacillus and decreased pH and Nugent score in women with symptomatic BV after four visits. Until now, there have been limited studies using probiotic vaginal administration in CC treatment, but some vaginal suppositories have reported high efficacy in HPV-positive women. A vaginal L. rhamnosus BMX therapy was implemented in 117 women with HPV and BV or vaginitis concomitantly after using an antibiotic (metronidazole) or antifungal (fluconazole) long-term. The study revealed that vaginal L. rhamnosus BMX could change abnormal cervical lesions and increase HPV clearance after long-term therapy [112].
Also, lacidophilin, a bacteriocin produced by lactic acid bacteria, has been considered a promising therapy. Lacidophilin can be an antibacterial factor by generating lactic acid and destroying the bacterial membrane [113]. The combination of lacidophilin and antitumor IFN-α2b was investigated for treating HPV-infected patients, with significant results in recovering vaginal microecology and inhibiting inflammatory factors [114].
The combination of probiotics and chemotherapy is currently being reported as a new approach to cervical cancer (CC) treatment. Previous studies have shown that using probiotic supplements can reduce the side effects or toxicity of chemotherapy [115]. For instance, cisplatin is a common therapy for CC; however, its toxicity affects normal cells and tissues. Negi et al. [116] combined cisplatin with probiotic-loaded pessaries (Lactobacillus rhamnosus) in a vaginal mouse model. They found a better outcome with fewer side effects of cisplatin and a reduced tumor volume in the treated group.
5.2.2. Probiotic Injection
A recent study reported the combination of a heat-killed preparation of L. casei and α-GalCer (an anticancer and NK T-cell stimulator) subcutaneous injections in a mouse model of CC. They found that splenocyte proliferation, lactate dehydrogenase, nitric oxide, and IFN-γ levels increased more in the combination therapy group than in the control group. Compared to Gardasil injection in the mouse model, the heat-killed preparation of L. casei and α-GalCer had a similar effect. These results could promise a new therapy for CC management [117].
5.2.3. Probiotics and Modulating the Gastrointestinal Problem of Cervical Cancer
Diarrhea is the most common side effect of radiotherapy in treating cervical cancer (CC) patients [118]. Previous studies have suggested that the supplementation of probiotics may prevent this gastrointestinal problem. For instance, Linn et al. [119] investigated the efficacy of Lactobacillus acidophilus LA-5 and Bifidobacterium animalis subsp. lactis BB-12 in 57 CC patients with diarrhea after radiotherapy. The study found that diarrhea symptoms in the mild-to-moderate group and the severe group were significantly reduced after three weeks of using probiotics.
VMT may improve CVM imbalance when transplanting vaginal fluid from a healthy person with high Lactobacillus abundance [120]. Lev-Sagie et al. [121] reported applying VMT to intractable and recurrent BV treatment. Five patients used VMT, and four had remissions with symptom improvement and no side effects after 5–21 months of transplantation. Although the number of patients was too small, this study could improve CVM dysbiosis and CC management in the future.
Overall, these findings suggest a significant role for probiotics in fighting CC. Nevertheless, further studies are required to understand the function of bacteriotherapy and its underlying mechanisms, particularly during CC treatment.
This review suggests a strong association between HPV infection and CVM status in cervical diseases. Accordingly, the dynamic interaction can cause an imbalanced cervicovaginal microenvironment, such as depletion of Lactobacillus and changes in the immune system and metabolism, which induce dysbiosis, enhance HPV persistence, and promote cervical carcinogenesis. However, this research is only beginning to understand the role of microorganisms in carcinogenesis. Further studies combining new molecular tools, or multiomics, should be considered to identify underlying mechanisms and provide insights into the interaction of microorganisms with immune and metabolic responses. Notwithstanding the relatively limited evidence, probiotics offer new opportunities for future therapy that could change the response to cancer treatment. Hence, continuing well-designed studies about the antitumor function of probiotics in managing CC patients is necessary.
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206.
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321.
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58.
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687.
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203.
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of Vaginal Lactobacilli as Probiotic Candidates. Sci. Rep. 2019, 9, 3355.
- D’Antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric and Genitourinary Cancers. Int. J. Mol. Sci. 2022, 23, 9664.
- Zhou, Z.; Hou, Y.; Qing, W.; Shi, Y.; Zhang, Y.; Chen, R.; Ou, J.; Zhou, H.; Chen, M. The Association of HPV Infection and Vaginal Microbiota of Reproductive Women in China: A Multicenter Cohort Study Protocol. Med. Microecol. 2023, 15, 100072.
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV Infection Alters Vaginal Microbiome through Down-Regulating Host Mucosal Innate Peptides Used by Lactobacilli as Amino Acid Sources. Nat. Commun. 2022, 13, 1076.
- Pourmollaei, S.; Barzegari, A.; Farshbaf-Khalili, A.; Nouri, M.; Fattahi, A.; Shahnazi, M.; Dittrich, R. Anticancer Effect of Bacteria on Cervical Cancer: Molecular Aspects and Therapeutic Implications. Life Sci. 2020, 246, 117413.
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal Microbiota Significantly Changed for HPV-Positive Women with High-Grade Squamous Intraepithelial Lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875.
- Kang, G.U.; Jung, D.R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.H. Potential Association between Vaginal Microbiota and Cervical Carcinogenesis in Korean Women: A Cohort Study. Microorganisms 2021, 9, 294.
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agent. Cancer 2022, 17, 53.
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics Enhance the Clearance of Human Papillomavirus-Related Cervical Lesions: A Prospective Controlled Pilot Study. Eur. J. Cancer Prev. 2013, 22, 46–51.
- Pierro, F.; Criscuolo, A.A.; Giudici, A.D.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial Francesco. Minerva Obstet. Gynecol. 2021, 73, 621–631.
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376.
- McBride, A.A. Human Papillomaviruses: Diversity, Infection and Host Interactions. Nat. Rev. Microbiol. 2021, 20, 95–108.
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229.
- Bzhalava, D.; Eklund, C.; Dillner, J. International Standardization and Classification of Human Papillomavirus Types. Virology 2015, 476, 341–344.
- McLaughlin-Daurbin, M.E.; Münger, K. Oncogenic Activities of Human Papillomaviruses Margaret. Virus Res. 2009, 143, 195–208.
- Alizon, S.; Murall, C.L.; Bravo, I.G. Why Human Papillomavirus Acute Infections Matter. Viruses 2017, 9, 293.
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221.
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818.
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic Role of HPV-Associated Early Proteins in Cervical Cancer: Molecular Pathways and Targeted Therapeutic Strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675.
- Squarzanti, D.F.; Sorrentino, R.; Landini, M.M.; Chiesa, A.; Pinato, S.; Rocchio, F.; Mattii, M.; Penengo, L.; Azzimonti, B. Human Papillomavirus Type 16 E6 and E7 Oncoproteins Interact with the Nuclear P53-Binding Protein 1 in an in Vitro Reconstructed 3D Epithelium: New Insights for the Virus-Induced DNA Damage Response. Virol. J. 2018, 15, 176.
- Fischer, M.; Uxa, S.; Stanko, C.; Magin, T.M.; Engeland, K. Human Papilloma Virus E7 Oncoprotein Abrogates the P53-P21-DREAM Pathway. Sci. Rep. 2017, 7, 2603.
- Giarrè, M.; Caldeira, S.; Malanchi, I.; Ciccolini, F.; Leão, M.J.; Tommasino, M. Induction of PRb Degradation by the Human Papillomavirus Type 16 E7 Protein Is Essential To Efficiently Overcome P16 INK4a -Imposed G 1 Cell Cycle Arrest. J. Virol. 2001, 75, 4705–4712.
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous Enumeration of Cancer and Immune Cell Types from Bulk Tumor Gene Expression Data. eLife 2017, 6, e26476.
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of Human Papillomavirus Type 16 into the Human Genome Correlates with a Selective Growth Advantage of Cells. J. Virol. 1995, 69, 2989–2997.
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642.
- Bienkowska-Haba, M.; Zwolinska, K.; Keiffer, T.; Scott, R.S.; Sapp, M. Human Papillomavirus Genome Copy Number Is Maintained by S-Phase Amplification, Genome Loss to the Cytosol during Mitosis, and Degradation in G1 Phase. J. Virol. 2023, 97, e01879-22.
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199.
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 Recognizes Cytosolic DsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 2009, 458, 514–518.
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19.
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 Promotes Cervical Cancer Progression by Upregulating PD-L1 in Immunomicroenvironment through STING-TBK1-NF-KB Pathway. Biomed. Pharmacother. 2020, 123, 109790.
- Yang, X.; Cheng, Y.; Li, C. The Role of TLRs in Cervical Cancer with HPV Infection: A Review. Signal Transduct. Target. Ther. 2017, 2, 17055.
- Chen, H.; Sun, H.; You, F.; Sun, W.; Zhou, X.; Chen, L.; Yang, J.; Wang, Y.; Tang, H.; Guan, Y.; et al. Activation of STAT6 by STING Is Critical for Antiviral Innate Immunity. Cell 2011, 147, 436–446.
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The Interactions between CGAS-STING Pathway and Pathogens. Signal Transduct. Target. Ther. 2020, 5, 91.
- Matsumiya, T.; Prescott, S.M.; Stafforini, D.M. IFN-ε Mediates TNF-α-Induced STAT1 Phosphorylation and Induction of Retinoic Acid-Inducible Gene-I in Human Cervical Cancer Cells. J. Immunol. 2007, 179, 4542–4549.
- Rattay, S.; Hufbauer, M.; Hagen, C.; Putschli, B.; Coch, C.; Akgül, B.; Hartmann, G. Human Beta Papillomavirus Type 8 E1 and E2 Proteins Suppress the Activation of the RIG-I-like Receptor MDA5. Viruses 2022, 14, 1361.
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-Λs Mediate Antiviral Protection through a Distinct Class II Cytokine Receptor Complex. Nat. Immunol. 2003, 4, 69–77.
- Cannella, F.; Scagnolari, C.; Selvaggi, C.; Stentella, P.; Recine, N.; Antonelli, G.; Pierangeli, A. Interferon Lambda 1 Expression in Cervical Cells Differs between Low-Risk and High-Risk Human Papillomavirus-Positive Women. Med. Microbiol. Immunol. 2014, 203, 177–184.
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-Risk Human Papillomaviruses Repress Constitutive Kappa Interferon Transcription via E6 To Prevent Pathogen Recognition Receptor and Antiviral-Gene Expression. J. Virol. 2011, 85, 11372–11380.
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923.
- Richards, K.H.; Wasson, C.W.; Watherston, O.; Doble, R.; Eric Blair, G.; Wittmann, M.; Macdonald, A. The Human Papillomavirus (HPV) E7 Protein Antagonises an Imiquimod-Induced Inflammatory Pathway in Primary Human Keratinocytes. Sci. Rep. 2015, 5, 12922.
- Vanguri, V.K. The Adaptive Immune System. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1–4. ISBN 9780123864567.
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Prim. 2016, 2, 16086.
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-Cell Markers in Cervical Carcinogenesis: A Systematic Review and Meta-Analysis. Br. J. Cancer 2021, 124, 831–841.
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222.
- Nakahara, T.; Kiyono, T. Interplay between NF-ΚB/Interferon Signaling and the Genome Replication of HPV. Future Virol. 2016, 11, 141–155.
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The Role of Nuclear Factor-Kappa B Signaling in Human Cervical Cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150.
- Horta, B.; Pereira, T.; Medeiros, R.; Cerqueira, F. Cervical Cancer Outcome and Tumor-Associated Macrophages: Research Evidence. Immuno 2022, 2, 460–468.
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving Immunosuppressive Microenvironment during Human Cervical Carcinogenesis. Mucosal Immunol. 2008, 1, 412–420.




