Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Uterine cervical cancer (CC) is a complex, multistep disease primarily linked to persistent infection with high-risk human papillomavirus (HR-HPV).
- cervicovaginal microbiome
- human papillomavirus
- cervical cancer
1. Introduction
Uterine cervical cancer (CC) remains a major public health problem, with a significant number of new cases and deaths annually [1]. Persistent human papillomavirus (HPV) infection, particularly high-risk genotypes (HR-HPV), is the most common cause of CC [2]. It is undeniable that HR-HPV infection is essential for CC formation and progression, but not sufficient alone. Moreover, drivers of the transition state between HPV acquisition, spacing, and persistence are poorly understood [3].
The cervicovaginal microbiome (CVM) has been considered essential to the female vaginal flora [4]. In most healthy women, the CVM is dominated by Lactobacillus spp., which benefits the host through symbiotic relationships [5][6]. Lactobacillus spp. depletion can lead to CVM dysbiosis, which may enhance tumor development through several mechanisms, such as promoting chronic inflammation, dysregulating the immune system, and producing genotoxins [7].
Mounting evidence has suggested an interaction between HPV, the CVM, and CC progression [8]. On the one hand, HPV infection can induce changes in the cervicovaginal microenvironment [9]. Consequently, this can lead to CVM dysbiosis and cancer. On the other hand, abnormalities in the cervicovaginal flora may change vaginal pH, release bacteriocin, and disrupt the mucosal layer. As a result, dysbiosis may contribute to HPV-related CC by interfering with HPV infection, binding, internalization, integration, gene expression, and telomerase activation [10]. Several studies have reported that women with HPV infection and CVM dysbiosis show high-grade squamous intraepithelial lesions (HSILs) or cancer [11][12], which may require closer follow-up and advanced treatment.
Bacteriotherapy, especially probiotics, has attracted enormous interest in CC prevention and treatment due to their antitumor activities [13][14][15][16]. Probiotics may become the best choice for controlling the complex interaction between the CVM, HPV, and cervical carcinogenesis.
2. Human Papillomavirus
2.1. Structure and Genome
HPV is the most common cause of sexually transmitted infections in women. This virus belongs to a group of nonenveloped and double-stranded DNA viruses [17]. The HPV genome is circular DNA containing eight open reading frames and divided into three encoded regions: an early region encoding a nonstructural protein (E1, E2, and E4–E7) for replication, a late region encoding viral capsid proteins (L1 and L2) for viral assembly, and a long control region (LCR) or upstream regulatory region [18].
There are >200 types of HPV, and ~40 genotypes infect the mucosal epithelium in the anogenital tract. Based on the association between these types and carcinogenicity, they are categorized into low-risk HPVs (HPV6, 11, 40, 42–44, and 54) and HR-HPVs (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) [19]. For example, HPV6 and HPV11, low-risk HPVs, are related to benign warts. In contrast, HPV16 and HPV18, HR-HPVs, can represent intraepithelial neoplasia with the potential for malignant progression [20].
2.2. Life Cycle of HPV
The most common HPV infections are caused by sexual intercourse when the vaginal and cervical epithelia are exposed to HPV through a microwound [21]. After entering basal keratinocytes, the viral genome moves to the nucleus and is maintained as episome DNA with a low copy number (50–100 copies per cell) [22].
In the early region, E2 is considered a regulator factor, whereas E6 and E7 are critical in inhibiting host tumor suppressor genes and oncogenic transformation [23]. Notably, the formation of an E1-E2 complex is required for the stable binding of the E1 helicase to the LCR ori site and controls the transcriptional levels of E6 and E7 viral oncogenes [24]. After integrating the HPV genome into the host DNA, the connection between E1 and E2 breaks, and E2 expression is lost. As a result, E6 and E7 expression is upregulated, which leads to the inactivation of tumor suppressor proteins p53 and pRb, respectively. This condition will promote malignant transformation in the cervix [25][26][27][28][29].
Until keratinocyte differentiation, the productive stage of the viral life cycle occurs by activating the late promoter (L1 and L2) and late viral gene expression (E4 and E5). Moreover, E1 and E2 expression increases HPV DNA amplification between 100 and 1000 episomal copies per cell [30][31]. Viral particles were then released from the uppermost layers of the stratified epithelium. There are ~3 weeks from infection to the release of the virus in which the virus can evade the immune system, and the appearance of lesions can occur after weeks to months [32].
2.3. HPV and Host Immune Responses
The host immune system, including the innate and adaptive immune systems, plays a vital role in clearing or controlling the infection and eliminating HPV-induced lesions.
2.3.1. HPV and Innate Immune Response
The essential step of the innate immune response is to detect pathogen-associated molecular patterns (PAMPs) by receptors located on the surface of sensor cells. During the early stages of HPV infection, pattern recognition receptors (PRRs) can recognize HPV and activate a cascade of antiviral signaling pathways to defend against its invasion. These pathogen sensors can detect DNA (known as DNA sensors: absent in melanoma 2 [33], interferon (IFN)-γ inducible protein 16 [34][35], Toll-like receptor 9 [36], and cyclic GMP-AMP synthase [37][38]) or RNA (known as RNA sensors: Toll-like receptor 3 [36], retinoic acid-inducible gene I [39], and melanoma differentiation-associated gene 5 [40]) in the cytoplasm or nucleus. One of the most important downstream reactions is the induction of IFN signaling, including types I and III IFNs, which stimulates the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signaling cascade, enhances IFN-stimulated gene (ISG) expression, and results in HPV clearance [41][42][43][44].
Several types of immune sentinels (sensor cells) exist in the innate system, such as dendritic cells, Langerhans cells, natural killer (NK) cells, and keratinocytes. Among them, keratinocytes target cells of HPV in early infection and play an essential role in response to the recognition between PRRs and PAMPs. Keratinocytes can detect various HPV-related patterns and secrete various cytokines and chemokines. These cytokines and chemokines promote immune responses and recruit more immune cells to the HPV-related microenvironment [17][45].
2.3.2. HPV and the Adaptive Immune Response
The adaptive immune system includes cell-mediated immune responses and antibody-mediated humoral immunity. The cell-mediated system is vital for destroying virus-infected cells, and the antibody-mediated system is responsible for clearing free pathogen particles from body fluids [46]. During HPV invasion, CD4+ T helper 1 (Th1) cells can detect HPV E6, E7, and E2 and induce cytotoxicity by activating CD8+ T cells and releasing interleukin (IL)-2 and IFN-γ [47]. It is agreed that whereas innate responses play a role in early HPV clearance, adaptive responses are essential to determining and eliminating HPV-induced lesions, particularly effector T cells [46].
A meta-analysis by Litwin et al. [48] supported the important role of T-cell populations in the outcome of cervical HPV infections. Helper and killer T cells are found at lower levels in low- and high-grade cervical lesions than in normal tissue, suggesting that the virus evades immune detection in patients with persistent lesions. Moreover, Foxp3+ and CD25+ regulatory T-cell (Treg) infiltration was high in precancerous HPV-related lesions, and longitudinal data showed improved outcomes with lower Treg levels.
2.3.3. HPV and Immune Suppression
Even if the immune reaction seems perfect and can clear most HPV infections, HPV has several immune evasion mechanisms and can facilitate cancer progression (Figure 1). First, most HPVs in the intraepithelial layer provide almost no viremia, release few proteins, induce less inflammation, and cause few host cell deaths [47][49]. Therefore, HPV can often prevent itself from being recognized by sensors.
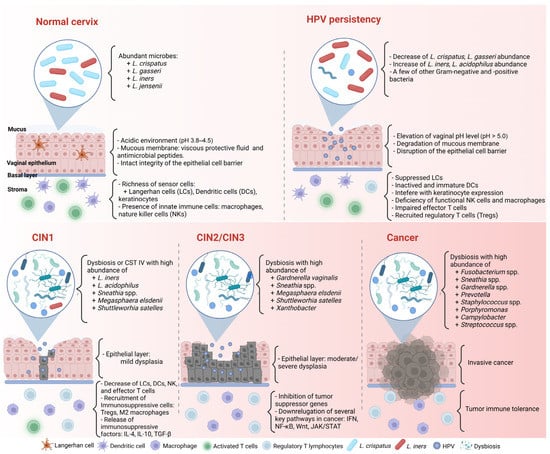
Figure 1. Complex association between HPV infection, the CVM, and cancer formation in the cervicovaginal microenvironment. CIN: cervical intraepithelial neoplasia. Created with BioRender.com (accessed on 30 March 2023).
Second, HPV can alter host gene, transcript factor, or protein expression to evade the immune response. For example, HPV blocks IFN signaling by inhibiting the activity of the IFN regulatory factors, interfering with the JAK/STAT signaling pathway, and downregulating the IFN-κ and ISG expressions. Moreover, HPV deregulates the activity of transcription factors, particularly nuclear factor-κB (NF-κB), to repress proinflammatory cytokine production. NF-κB is one of the members of the NF-κB family responsible for controlling cell proliferation and apoptosis through the NF-κB/IFN signaling pathway. HR-HPV can eradicate the inhibitory effect of the immune system and lead to persistent infection by decreasing NF-κB activation [50][51].
Third, the HPV-related microenvironment can inhibit the infiltration of helper and cytotoxic T cells while recruiting more immunosuppressive cells, such as M2 macrophages, myeloid-derived suppressor cells (MDSCs), and Tregs [48][52]. Tregs are related to downregulated responses to exogenous antigens. When Tregs are recruited to a tumor, they suppress the adaptive immune response and promote HPV-related lesion formation. M2 macrophages and MDSCs can enhance HPV-associated tumor progression by inhibiting CD4+ and CD8+ T cells, attracting Tregs, and producing immunosuppressive factors such as IL-10 and transforming growth factor-β (TGF-β) [53].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11061417
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206.
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321.
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The Vaginal Microbiota, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia: What Do We Know and Where Are We Going Next? Microbiome 2016, 4, 58.
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687.
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 2020, 19, 203.
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of Vaginal Lactobacilli as Probiotic Candidates. Sci. Rep. 2019, 9, 3355.
- D’Antonio, D.L.; Marchetti, S.; Pignatelli, P.; Piattelli, A.; Curia, M.C. The Oncobiome in Gastroenteric and Genitourinary Cancers. Int. J. Mol. Sci. 2022, 23, 9664.
- Zhou, Z.; Hou, Y.; Qing, W.; Shi, Y.; Zhang, Y.; Chen, R.; Ou, J.; Zhou, H.; Chen, M. The Association of HPV Infection and Vaginal Microbiota of Reproductive Women in China: A Multicenter Cohort Study Protocol. Med. Microecol. 2023, 15, 100072.
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV Infection Alters Vaginal Microbiome through Down-Regulating Host Mucosal Innate Peptides Used by Lactobacilli as Amino Acid Sources. Nat. Commun. 2022, 13, 1076.
- Pourmollaei, S.; Barzegari, A.; Farshbaf-Khalili, A.; Nouri, M.; Fattahi, A.; Shahnazi, M.; Dittrich, R. Anticancer Effect of Bacteria on Cervical Cancer: Molecular Aspects and Therapeutic Implications. Life Sci. 2020, 246, 117413.
- Guo, C.; Dai, W.; Zhou, Q.; Gui, L.; Cai, H.; Wu, D.; Hou, J.; Li, C.; Li, S.; Du, H.; et al. Cervicovaginal Microbiota Significantly Changed for HPV-Positive Women with High-Grade Squamous Intraepithelial Lesion. Front. Cell. Infect. Microbiol. 2022, 12, 973875.
- Kang, G.U.; Jung, D.R.; Lee, Y.H.; Jeon, S.Y.; Han, H.S.; Chong, G.O.; Shin, J.H. Potential Association between Vaginal Microbiota and Cervical Carcinogenesis in Korean Women: A Cohort Study. Microorganisms 2021, 9, 294.
- Dellino, M.; Cascardi, E.; Laganà, A.S.; Di Vagno, G.; Malvasi, A.; Zaccaro, R.; Maggipinto, K.; Cazzato, G.; Scacco, S.; Tinelli, R.; et al. Lactobacillus Crispatus M247 Oral Administration: Is It Really an Effective Strategy in the Management of Papillomavirus-Infected Women? Infect. Agent. Cancer 2022, 17, 53.
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics Enhance the Clearance of Human Papillomavirus-Related Cervical Lesions: A Prospective Controlled Pilot Study. Eur. J. Cancer Prev. 2013, 22, 46–51.
- Pierro, F.; Criscuolo, A.A.; Giudici, A.D.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral Administration of Lactobacillus Crispatus M247 to Papillomavirus-Infected Women: Results of a Preliminary, Uncontrolled, Open Trial Francesco. Minerva Obstet. Gynecol. 2021, 73, 621–631.
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of Lactobacilli and Lactoferrin in the Mucosal Cervicovaginal Defense. Front. Immunol. 2018, 9, 376.
- McBride, A.A. Human Papillomaviruses: Diversity, Infection and Host Interactions. Nat. Rev. Microbiol. 2021, 20, 95–108.
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229.
- Bzhalava, D.; Eklund, C.; Dillner, J. International Standardization and Classification of Human Papillomavirus Types. Virology 2015, 476, 341–344.
- McLaughlin-Daurbin, M.E.; Münger, K. Oncogenic Activities of Human Papillomaviruses Margaret. Virus Res. 2009, 143, 195–208.
- Alizon, S.; Murall, C.L.; Bravo, I.G. Why Human Papillomavirus Acute Infections Matter. Viruses 2017, 9, 293.
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221.
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818.
- Bhattacharjee, R.; Das, S.S.; Biswal, S.S.; Nath, A.; Das, D.; Basu, A.; Malik, S.; Kumar, L.; Kar, S.; Singh, S.K.; et al. Mechanistic Role of HPV-Associated Early Proteins in Cervical Cancer: Molecular Pathways and Targeted Therapeutic Strategies. Crit. Rev. Oncol. Hematol. 2022, 174, 103675.
- Squarzanti, D.F.; Sorrentino, R.; Landini, M.M.; Chiesa, A.; Pinato, S.; Rocchio, F.; Mattii, M.; Penengo, L.; Azzimonti, B. Human Papillomavirus Type 16 E6 and E7 Oncoproteins Interact with the Nuclear P53-Binding Protein 1 in an in Vitro Reconstructed 3D Epithelium: New Insights for the Virus-Induced DNA Damage Response. Virol. J. 2018, 15, 176.
- Fischer, M.; Uxa, S.; Stanko, C.; Magin, T.M.; Engeland, K. Human Papilloma Virus E7 Oncoprotein Abrogates the P53-P21-DREAM Pathway. Sci. Rep. 2017, 7, 2603.
- Giarrè, M.; Caldeira, S.; Malanchi, I.; Ciccolini, F.; Leão, M.J.; Tommasino, M. Induction of PRb Degradation by the Human Papillomavirus Type 16 E7 Protein Is Essential To Efficiently Overcome P16 INK4a -Imposed G 1 Cell Cycle Arrest. J. Virol. 2001, 75, 4705–4712.
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous Enumeration of Cancer and Immune Cell Types from Bulk Tumor Gene Expression Data. eLife 2017, 6, e26476.
- Jeon, S.; Allen-Hoffmann, B.L.; Lambert, P.F. Integration of Human Papillomavirus Type 16 into the Human Genome Correlates with a Selective Growth Advantage of Cells. J. Virol. 1995, 69, 2989–2997.
- Amador-Molina, A.; Hernández-Valencia, J.F.; Lamoyi, E.; Contreras-Paredes, A.; Lizano, M. Role of Innate Immunity against Human Papillomavirus (HPV) Infections and Effect of Adjuvants in Promoting Specific Immune Response. Viruses 2013, 5, 2624–2642.
- Bienkowska-Haba, M.; Zwolinska, K.; Keiffer, T.; Scott, R.S.; Sapp, M. Human Papillomavirus Genome Copy Number Is Maintained by S-Phase Amplification, Genome Loss to the Cytosol during Mitosis, and Degradation in G1 Phase. J. Virol. 2023, 97, e01879-22.
- Wakabayashi, R.; Nakahama, Y.; Nguyen, V.; Espinoza, J.L. The Host-Microbe Interplay in Human Papillomavirus-Induced Carcinogenesis. Microorganisms 2019, 7, 199.
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 Recognizes Cytosolic DsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 2009, 458, 514–518.
- Scott, M.L.; Woodby, B.L.; Ulicny, J.; Raikhy, G.; Orr, A.W.; Songock, W.K. Human Papillomavirus 16 E5 Inhibits Interferon Signaling and Supports Episomal Viral Maintenance. J. Virol. 2020, 94, e01582-19.
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 Promotes Cervical Cancer Progression by Upregulating PD-L1 in Immunomicroenvironment through STING-TBK1-NF-KB Pathway. Biomed. Pharmacother. 2020, 123, 109790.
- Yang, X.; Cheng, Y.; Li, C. The Role of TLRs in Cervical Cancer with HPV Infection: A Review. Signal Transduct. Target. Ther. 2017, 2, 17055.
- Chen, H.; Sun, H.; You, F.; Sun, W.; Zhou, X.; Chen, L.; Yang, J.; Wang, Y.; Tang, H.; Guan, Y.; et al. Activation of STAT6 by STING Is Critical for Antiviral Innate Immunity. Cell 2011, 147, 436–446.
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The Interactions between CGAS-STING Pathway and Pathogens. Signal Transduct. Target. Ther. 2020, 5, 91.
- Matsumiya, T.; Prescott, S.M.; Stafforini, D.M. IFN-ε Mediates TNF-α-Induced STAT1 Phosphorylation and Induction of Retinoic Acid-Inducible Gene-I in Human Cervical Cancer Cells. J. Immunol. 2007, 179, 4542–4549.
- Rattay, S.; Hufbauer, M.; Hagen, C.; Putschli, B.; Coch, C.; Akgül, B.; Hartmann, G. Human Beta Papillomavirus Type 8 E1 and E2 Proteins Suppress the Activation of the RIG-I-like Receptor MDA5. Viruses 2022, 14, 1361.
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-Λs Mediate Antiviral Protection through a Distinct Class II Cytokine Receptor Complex. Nat. Immunol. 2003, 4, 69–77.
- Cannella, F.; Scagnolari, C.; Selvaggi, C.; Stentella, P.; Recine, N.; Antonelli, G.; Pierangeli, A. Interferon Lambda 1 Expression in Cervical Cells Differs between Low-Risk and High-Risk Human Papillomavirus-Positive Women. Med. Microbiol. Immunol. 2014, 203, 177–184.
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-Risk Human Papillomaviruses Repress Constitutive Kappa Interferon Transcription via E6 To Prevent Pathogen Recognition Receptor and Antiviral-Gene Expression. J. Virol. 2011, 85, 11372–11380.
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923.
- Richards, K.H.; Wasson, C.W.; Watherston, O.; Doble, R.; Eric Blair, G.; Wittmann, M.; Macdonald, A. The Human Papillomavirus (HPV) E7 Protein Antagonises an Imiquimod-Induced Inflammatory Pathway in Primary Human Keratinocytes. Sci. Rep. 2015, 5, 12922.
- Vanguri, V.K. The Adaptive Immune System. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1–4. ISBN 9780123864567.
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Prim. 2016, 2, 16086.
- Litwin, T.R.; Irvin, S.R.; Chornock, R.L.; Sahasrabuddhe, V.V.; Stanley, M.; Wentzensen, N. Infiltrating T-Cell Markers in Cervical Carcinogenesis: A Systematic Review and Meta-Analysis. Br. J. Cancer 2021, 124, 831–841.
- Stanley, M.A. Epithelial Cell Responses to Infection with Human Papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222.
- Nakahara, T.; Kiyono, T. Interplay between NF-ΚB/Interferon Signaling and the Genome Replication of HPV. Future Virol. 2016, 11, 141–155.
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The Role of Nuclear Factor-Kappa B Signaling in Human Cervical Cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150.
- Horta, B.; Pereira, T.; Medeiros, R.; Cerqueira, F. Cervical Cancer Outcome and Tumor-Associated Macrophages: Research Evidence. Immuno 2022, 2, 460–468.
- Kobayashi, A.; Weinberg, V.; Darragh, T.; Smith-McCune, K. Evolving Immunosuppressive Microenvironment during Human Cervical Carcinogenesis. Mucosal Immunol. 2008, 1, 412–420.
This entry is offline, you can click here to edit this entry!
