
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pierluigi Scalia | -- | 2024 | 2023-06-12 16:50:43 | | | |
| 2 | Dean Liu | -6 word(s) | 2018 | 2023-06-13 03:16:19 | | | | |
| 3 | Dean Liu | -3 word(s) | 2015 | 2023-06-20 02:36:02 | | |
Video Upload Options
Regulation of the human IGF2 gene displays multiple layers of control, which secures a genetically and epigenetically predetermined gene expression pattern throughout embryonal growth and postnatal life. These predominantly nuclear regulatory mechanisms converge on the function of the IGF2-H19 gene cluster on Chromosome 11 and ultimately affect IGF2 gene expression. Deregulation of such control checkpoints leads to the enhancement of IGF2 gene transcription and/or transcript stabilization, ultimately leading to IGF-II peptide overproduction. This type of anomaly is responsible for the effects observed in terms of both abnormal fetal growth and increased cell proliferation, typically observed in pediatric overgrowth syndromes and cancer.
1. IGF2 Gene Regulation at the Promoter and Transcript Level: An Unexploited View
2. The Human IGF2 Gene Structure
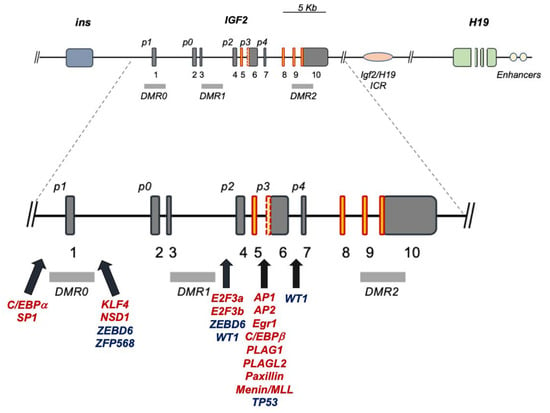
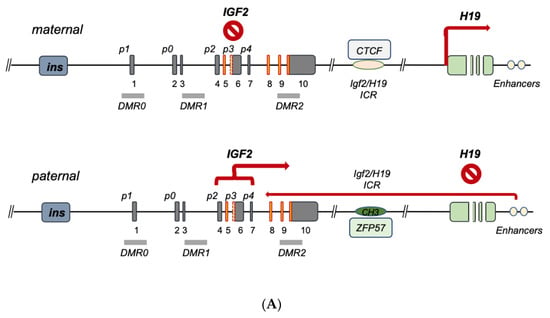
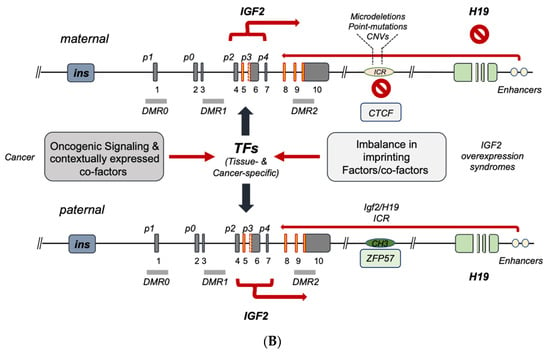
3. IGF2 Gene Regulation during Development and IGF2 Overexpression Syndromes
| Imprinting Factor | Key Feature | Reference(s) |
|---|---|---|
| CTCF | binds maternal ICR and insulates IGF2-p activity |
[34] |
| Cohesin | Cohesin is required for chromatin function at the H19/IGF2 locus | [41] |
| EZH2 | CH3-transferase component of PRC2 | [40] |
| SUZ12 | PRC2 component enabling ICR imprinting |
[37] |
| Sox2/Oct3–4 | CTCF-like effect | [35] |
| Vigilin | ICR imprinting effect via CTCF binding | [38] |
| ZFP57 | Binds paternal ICR and maintains methylated status | [28] |
4. IGF2 Gene Transcriptional Control in Cancer
| Promoter Usage | Imprinting Control | Reference(s) |
|---|---|---|
| IGF2-p0 | Not imprinted Mostly active in fetal placenta |
[14] |
| IGF2-p1 | Not imprinted—mostly active in postnatal Liver |
[56][57] |
| IGF2-p2 | Imprinted- Mostly active during Fetal growth |
[58] |
| IGF2-p3 & IGF2-p3/p4 (*) |
Imprinted- Mostly active during Fetal growth, Widely reactivated in cancer |
[50][56][57][59] |
References
- DeChiara, T.M.; Robertson, E.J.; Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 1991, 64, 849–859.
- Bell, A.C.; Felsenfeld, G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 2000, 405, 482–485.
- Thorvaldsen, J.L.; Duran, K.L.; Bartolomei, M.S. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 1998, 12, 3693–3702.
- Sasaki, H.; Jones, P.A.; Chaillet, J.R.; Ferguson-Smith, A.C.; Barton, S.C.; Reik, W.; Surani, M.A. Parental imprinting: Potentially active chromatin of the repressed maternal allele of the mouse insulin-like growth factor II (Igf2) gene. Genes Dev. 1992, 6, 1843–1856.
- Morison, I.M.; Becroft, D.M.; Taniguchi, T.; Woods, C.G.; Reeve, A.E. Somatic overgrowth associated with overexpression of insulin-like growth factor II. Nat. Med. 1996, 2, 311–316.
- Ogawa, O.; Mishina, M.; Yoshida, O. Activation of imprinted genes in human carcinogenesis. Nihon rinsho. Jpn. J. Clin. Med. 1995, 53, 1009–1016.
- Taniguchi, T.; Sullivan, M.J.; Ogawa, O.; Reeve, A.E. Epigenetic changes encompassing the IGF2/H19 locus associated with relaxation of IGF2 imprinting and silencing of H19 in Wilms tumor. Proc. Natl. Acad. Sci. USA 1995, 92, 2159–2163.
- Reik, W.; Constancia, M.; Dean, W.; Davies, K.; Bowden, L.; Murrell, A.; Feil, R.; Walter, J.; Kelsey, G. Igf2 imprinting in development and disease. Int. J. Dev. Biol. 2000, 44, 145–150.
- Sussenbach, J.S.; Rodenburg, R.J.T.; Scheper, W.; Holthuizen, P. Transcriptional and Post-Transcriptional Regulation of the Human IGF-II Gene Expression. In Current Directions in Insulin-Like Growth Factor Research; Le Roith, D., Raizada, M.K., Eds.; Springer US: Boston, MA, USA, 1993; pp. 63–71.
- Mineo, R.; Fichera, E.; Liang, S.J.; Fujita-Yamaguchi, Y. Promoter usage for insulin-like growth factor-II in cancerous and benign human breast, prostate, and bladder tissues, and confirmation of a 10th exon. Biochem. Biophys. Res. Commun. 2000, 268, 886–892.
- Baral, K.; Rotwein, P. The insulin-like growth factor 2 gene in mammals: Organizational complexity within a conserved locus. PLoS ONE 2019, 14, e0219155.
- Holthuizen, P.; Van Dijk, M.A.; Rodenburg, R.J.; Koonen-Reemst, A.M.; Sussenbach, J.S. Transcriptional regulation of the major promoters of the human IGF-II gene. Mol. Reprod. Dev. 1993, 35, 391–393.
- Constancia, M.; Hemberger, M.; Hughes, J.; Dean, W.; Ferguson-Smith, A.; Fundele, R.; Stewart, F.; Kelsey, G.; Fowden, A.; Sibley, C.; et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002, 417, 945–948.
- Monk, D.; Sanches, R.; Arnaud, P.; Apostolidou, S.; Hills, F.A.; Abu-Amero, S.; Murrell, A.; Friess, H.; Reik, W.; Stanier, P.; et al. Imprinting of IGF2 P0 transcript and novel alternatively spliced INS-IGF2 isoforms show differences between mouse and human. Hum. Mol. Genet. 2006, 15, 1259–1269.
- Christofori, G.; Naik, P.; Hanahan, D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 1994, 369, 414–418.
- Scalia, P.; Giordano, A.; Williams, S.J. The IGF-II-Insulin Receptor Isoform-A Autocrine Signal in Cancer: Actionable Perspectives. Cancers 2020, 12, 366.
- Mercader, J.M.; Liao, R.G.; Bell, A.D.; Dymek, Z.; Estrada, K.; Tukiainen, T.; Huerta-Chagoya, A.; Moreno-Macias, H.; Jablonski, K.A.; Hanson, R.L.; et al. A Loss-of-Function Splice Acceptor Variant in IGF2 Is Protective for Type 2 Diabetes. Diabetes 2017, 66, 2903–2914.
- Davies, K.; Bowden, L.; Smith, P.; Dean, W.; Hill, D.; Furuumi, H.; Sasaki, H.; Cattanach, B.; Reik, W. Disruption of mesodermal enhancers for Igf2 in the minute mutant. Development 2002, 129, 1657–1668.
- Murrell, A.; Heeson, S.; Cooper, W.N.; Douglas, E.; Apostolidou, S.; Moore, G.E.; Maher, E.R.; Reik, W. An association between variants in the IGF2 gene and Beckwith-Wiedemann syndrome: Interaction between genotype and epigenotype. Hum. Mol. Genet. 2004, 13, 247–255.
- Charalambous, M.; Menheniott, T.R.; Bennett, W.R.; Kelly, S.M.; Dell, G.; Dandolo, L.; Ward, A. An enhancer element at the Igf2/H19 locus drives gene expression in both imprinted and non-imprinted tissues. Dev. Biol. 2004, 271, 488–497.
- Kurukuti, S.; Tiwari, V.K.; Tavoosidana, G.; Pugacheva, E.; Murrell, A.; Zhao, Z.; Lobanenkov, V.; Reik, W.; Ohlsson, R. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl. Acad. Sci. USA 2006, 103, 10684–10689.
- Murphy, S.K.; Erginer, E.; Huang, Z.; Visco, Z.; Hoyo, C. Genotype-Epigenotype Interaction at the IGF2 DMR. Genes 2015, 6, 777–789.
- Papulino, C.; Chianese, U.; Nicoletti, M.M.; Benedetti, R.; Altucci, L. Preclinical and Clinical Epigenetic-Based Reconsideration of Beckwith-Wiedemann Syndrome. Front. Genet. 2020, 11, 563718.
- Hark, A.T.; Schoenherr, C.J.; Katz, D.J.; Ingram, R.S.; Levorse, J.M.; Tilghman, S.M. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 2000, 405, 486–489.
- Pant, V.; Mariano, P.; Kanduri, C.; Mattsson, A.; Lobanenkov, V.; Heuchel, R.; Ohlsson, R. The nucleotides responsible for the direct physical contact between the chromatin insulator protein CTCF and the H19 imprinting control region manifest parent of origin-specific long-distance insulation and methylation-free domains. Genes Dev. 2003, 17, 586–590.
- Lewis, A.; Murrell, A. Genomic imprinting: CTCF protects the boundaries. Curr. Biol. 2004, 14, R284–R286.
- Zhang, H.; Niu, B.; Hu, J.F.; Ge, S.; Wang, H.; Li, T.; Ling, J.; Steelman, B.N.; Qian, G.; Hoffman, A.R. Interruption of intrachromosomal looping by CCCTC binding factor decoy proteins abrogates genomic imprinting of human insulin-like growth factor II. J. Cell Biol. 2011, 193, 475–487.
- Riso, V.; Cammisa, M.; Kukreja, H.; Anvar, Z.; Verde, G.; Sparago, A.; Acurzio, B.; Lad, S.; Lonardo, E.; Sankar, A.; et al. ZFP57 maintains the parent-of-origin-specific expression of the imprinted genes and differentially affects non-imprinted targets in mouse embryonic stem cells. Nucleic Acids Res. 2016, 44, 8165–8178.
- Sparago, A.; Cerrato, F.; Vernucci, M.; Ferrero, G.B.; Silengo, M.C.; Riccio, A. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat. Genet. 2004, 36, 958–960.
- Poole, R.L.; Leith, D.J.; Docherty, L.E.; Shmela, M.E.; Gicquel, C.; Splitt, M.; Temple, I.K.; Mackay, D.J. Beckwith-Wiedemann syndrome caused by maternally inherited mutation of an OCT-binding motif in the IGF2/H19-imprinting control region, ICR1. Eur. J. Hum. Genet. 2012, 20, 240–243.
- Abi Habib, W.; Brioude, F.; Azzi, S.; Salem, J.; Das Neves, C.; Personnier, C.; Chantot-Bastaraud, S.; Keren, B.; Le Bouc, Y.; Harbison, M.D.; et al. 11p15 ICR1 Partial Deletions Associated with IGF2/H19 DMR Hypomethylation and Silver-Russell Syndrome. Hum. Mutat. 2017, 38, 105–111.
- Haruta, M.; Arai, Y.; Sugawara, W.; Watanabe, N.; Honda, S.; Ohshima, J.; Soejima, H.; Nakadate, H.; Okita, H.; Hata, J.; et al. Duplication of paternal IGF2 or loss of maternal IGF2 imprinting occurs in half of Wilms tumors with various structural WT1 abnormalities. Genes Chromosomes Cancer 2008, 47, 712–727.
- Hubertus, J.; Lacher, M.; Rottenkolber, M.; Müller-Höcker, J.; Berger, M.; Stehr, M.; von Schweinitz, D.; Kappler, R. Altered expression of imprinted genes in Wilms tumors. Oncol. Rep. 2011, 25, 817–823.
- Li, T.; Hu, J.F.; Qiu, X.; Ling, J.; Chen, H.; Wang, S.; Hou, A.; Vu, T.H.; Hoffman, A.R. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex 2 intrachromosomal loop. Mol. Cell. Biol. 2008, 28, 6473–6482.
- Hori, N.; Yamane, M.; Kouno, K.; Sato, K. Induction of DNA demethylation depending on two sets of Sox2 and adjacent Oct3/4 binding sites (Sox-Oct motifs) within the mouse H19/insulin-like growth factor 2 (Igf2) imprinted control region. J. Biol. Chem. 2012, 287, 44006–44016.
- Higashimoto, K.; Jozaki, K.; Kosho, T.; Matsubara, K.; Fuke, T.; Yamada, D.; Yatsuki, H.; Maeda, T.; Ohtsuka, Y.; Nishioka, K.; et al. A novel de novo point mutation of the OCT-binding site in the IGF2/H19-imprinting control region in a Beckwith-Wiedemann syndrome patient. Clin. Genet. 2014, 86, 539–544.
- Wang, H.; Ge, S.; Qian, G.; Li, W.; Cui, J.; Wang, G.; Hoffman, A.R.; Hu, J.F. Restoration of IGF2 imprinting by polycomb repressive complex 2 docking factor SUZ12 in colon cancer cells. Exp. Cell Res. 2015, 338, 214–221.
- Liu, Q.; Yang, B.; Xie, X.; Wei, L.; Liu, W.; Yang, W.; Ge, Y.; Zhu, Q.; Zhang, J.; Jiang, L.; et al. Vigilin interacts with CCCTC-binding factor (CTCF) and is involved in CTCF-dependent regulation of the imprinted genes Igf2 and H19. FEBS J. 2014, 281, 2713–2725.
- Hu, J.F.; Oruganti, H.; Vu, T.H.; Hoffman, A.R. The role of histone acetylation in the allelic expression of the imprinted human insulin-like growth factor II gene. Biochem. Biophys. Res. Commun. 1998, 251, 403–408.
- Li, T.; Chen, H.; Li, W.; Cui, J.; Wang, G.; Hu, X.; Hoffman, A.R.; Hu, J. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum. Mol. Genet. 2014, 23, 117–128.
- Nativio, R.; Wendt, K.S.; Ito, Y.; Huddleston, J.E.; Uribe-Lewis, S.; Woodfine, K.; Krueger, C.; Reik, W.; Peters, J.M.; Murrell, A. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet. 2009, 5, e1000739.
- Issa, J.P.; Vertino, P.M.; Boehm, C.D.; Newsham, I.F.; Baylin, S.B. Switch from monoallelic to biallelic human IGF2 promoter methylation during aging and carcinogenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 11757–11762.
- Cui, H.; Cruz-Correa, M.; Giardiello, F.M.; Hutcheon, D.F.; Kafonek, D.R.; Brandenburg, S.; Wu, Y.; He, X.; Powe, N.R.; Feinberg, A.P. Loss of IGF2 imprinting: A potential marker of colorectal cancer risk. Science 2003, 299, 1753–1755.
- Bjornsson, H.T.; Brown, L.J.; Fallin, M.D.; Rongione, M.A.; Bibikova, M.; Wickham, E.; Fan, J.B.; Feinberg, A.P. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J. Natl. Cancer Inst. 2007, 99, 1270–1273.
- Lu, L.; Cai, M.; Peng, M.; Wang, F.; Zhai, X. miR-491-5p functions as a tumor suppressor by targeting IGF2 in colorectal cancer. Cancer Manag. Res. 2019, 11, 1805–1816.
- Li, X.; Adam, G.; Cui, H.; Sandstedt, B.; Ohlsson, R.; Ekstrom, T.J. Expression, promoter usage and parental imprinting status of insulin-like growth factor II (IGF2) in human hepatoblastoma: Uncoupling of IGF2 and H19 imprinting. Oncogene 1995, 11, 221–229.
- Douc-Rasy, S.; Barrois, M.; Fogel, S.; Ahomadegbe, J.C.; Stéhelin, D.; Coll, J.; Riou, G. High incidence of loss of heterozygosity and abnormal imprinting of H19 and IGF2 genes in invasive cervical carcinomas. Uncoupling of H19 and IGF2 expression and biallelic hypomethylation of H19. Oncogene 1996, 12, 423–430.
- Vu, T.H.; Hoffman, A. Alterations in the promoter-specific imprinting of the insulin-like growth factor-II gene in Wilms’ tumor. J. Biol. Chem. 1996, 271, 9014–9023.
- Hodzic, D.; Frey, B.; Marechal, D.; Scarcez, T.; Grooteclaes, M.; Winkler, R. Cloning of breakpoints in and downstream the IGF2 gene that are associated with overexpression of IGF2 transcripts in colorectal tumours. Oncogene 1999, 18, 4710–4717.
- Eriksson, T.; Frisk, T.; Gray, S.G.; von Schweinitz, D.; Pietsch, T.; Larsson, C.; Sandstedt, B.; Ekström, T.J. Methylation Changes in the Human IGF2 P3 Promoter Parallel IGF2 Expression in the Primary Tumor, Established Cell Line, and Xenograft of a Human Hepatoblastoma. Exp. Cell Res. 2001, 270, 88–95.
- Kim, S.J.; Park, S.E.; Lee, C.; Lee, S.Y.; Jo, J.H.; Kim, J.M.; Oh, Y.K. Alterations in promoter usage and expression levels of insulin-like growth factor-II and H19 genes in cervical carcinoma exhibiting biallelic expression of IGF-II. Biochim. Biophys. Acta 2002, 1586, 307–315.
- Yu, Y.; Wylie-Sears, J.; Boscolo, E.; Mulliken, J.B.; Bischoff, J. Genomic imprinting of IGF2 is maintained in infantile hemangioma despite its high level of expression. Mol. Med. 2004, 10, 117–123.
- Murphy, S.K.; Huang, Z.; Wen, Y.; Spillman, M.A.; Whitaker, R.S.; Simel, L.R.; Nichols, T.D.; Marks, J.R.; Berchuck, A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol. Cancer Res. 2006, 4, 283–292.
- Qian, B.; Katsaros, D.; Lu, L.; Canuto, E.M.; Benedetto, C.; Beeghly-Fadiel, A.; Yu, H. IGF-II promoter specific methylation and expression in epithelial ovarian cancer and their associations with disease characteristics. Oncol. Rep. 2011, 25, 203–213.
- Küffer, S.; Gutting, T.; Belharazem, D.; Sauer, C.; Michel, M.S.; Marx, A.; Trojan, L.; Ströbel, P. Insulin-like growth factor 2 expression in prostate cancer is regulated by promoter-specific methylation. Mol. Oncol. 2018, 12, 256–266.
- Nardone, G.; Romano, M.; Calabrò, A.; Pedone, P.V.; de Sio, I.; Persico, M.; Budillon, G.; Bruni, C.B.; Riccio, A.; Zarrilli, R. Activation of fetal promoters of insulin-like growth factors II gene in hepatitis C virus-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Hepatology 1996, 23, 1304–1312.
- Li, X.; Nong, Z.; Ekström, C.; Larsson, E.; Nordlinder, H.; Hofmann, W.J.; Trautwein, C.; Odenthal, M.; Dienes, H.P.; Ekström, T.J.; et al. Disrupted IGF2 promoter control by silencing of promoter P1 in human hepatocellular carcinoma. Cancer Res. 1997, 57, 2048–2054.
- Lui, J.C.; Baron, J. Evidence that Igf2 down-regulation in postnatal tissues and up-regulation in malignancies is driven by transcription factor E2f3. Proc. Natl. Acad. Sci. USA 2013, 110, 6181–6186.
- Li, Y.; Meng, G.; Huang, L.; Guo, Q.N. Hypomethylation of the P3 promoter is associated with up-regulation of IGF2 expression in human osteosarcoma. Hum. Pathol. 2009, 40, 1441–1447.




