Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David C Hay | -- | 1588 | 2023-06-12 12:41:52 | | | |
| 2 | Wendy Huang | Meta information modification | 1588 | 2023-06-12 13:58:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sinton, M.; Kasarinaite, A.; Saunders, P.T.K.; Hay, D.C. Immune System's Role in Liver Biology and Metabolism. Encyclopedia. Available online: https://encyclopedia.pub/entry/45449 (accessed on 13 January 2026).
Sinton M, Kasarinaite A, Saunders PTK, Hay DC. Immune System's Role in Liver Biology and Metabolism. Encyclopedia. Available at: https://encyclopedia.pub/entry/45449. Accessed January 13, 2026.
Sinton, Matthew, Alvile Kasarinaite, Philippa T. K. Saunders, David C. Hay. "Immune System's Role in Liver Biology and Metabolism" Encyclopedia, https://encyclopedia.pub/entry/45449 (accessed January 13, 2026).
Sinton, M., Kasarinaite, A., Saunders, P.T.K., & Hay, D.C. (2023, June 12). Immune System's Role in Liver Biology and Metabolism. In Encyclopedia. https://encyclopedia.pub/entry/45449
Sinton, Matthew, et al. "Immune System's Role in Liver Biology and Metabolism." Encyclopedia. Web. 12 June, 2023.
Copy Citation
The liver is a remarkable organ, which coordinates a multitude of critical functions, whilst retaining the ability to dramatically remodel and regenerate damaged tissue. The liver is composed of four lobes which are subdivided into lobule structures. These are hexagonal in appearance, with each corner displaying the portal triad that consists of the portal vein, bile duct and hepatic artery. Although the liver is an exceptionally regenerative organ, chronic damage may result in scar tissue formation. This does not only have consequences for organ function but is also a major barrier for liver tissue remodeling and regeneration.
liver
hepatocyte
immune response
metabolism
immune system
1. Introduction
In its healthy state, the liver functions as an immune sentinel, sampling the blood that enters it via the hepatic portal vein, before it reaches the spleen or lymph nodes. This blood is rich in nutrients, as well as any pathogen-derived molecules that are in circulation, such as lipopolysaccharide (LPS). Since the liver filters all of the blood, it is in a prime position to detect these molecules and sound the alarm to the immune system. It is, therefore, unsurprising that damage to the liver leads to a robust immune response, by both the innate and adaptive arms of the immune system (Figure 1). NAFLD leads to lipotoxicity within the liver, which, in turn, causes hepatocytes to become stressed or die, and this process generates inflammatory factors called damage-associated molecular patterns (DAMPs) [1], which are able to trigger activation of the immune system [2]. Whilst the precise mechanisms leading to the progression from NAFLD to NASH remain unclear, there is evidence to suggest that these are, in part, immunological in nature.
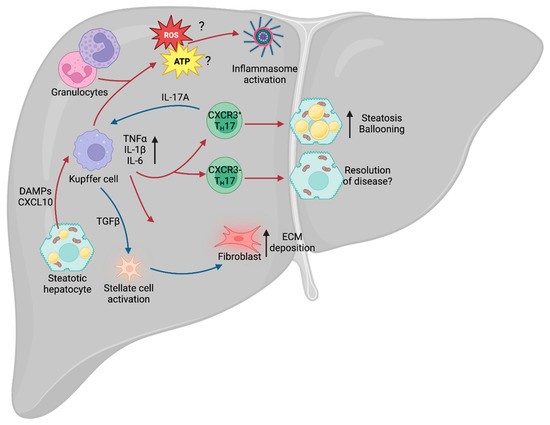
Figure 1. Liver immunity. Schematic of the broad immune changes occurring in the liver during NAFLD. Hepatic steatosis causes the release of DAMPs and CXCL10, leading to activation of the liver-resident macrophages (Kupffer cells). Kupffer cells, in turn, recruit the adaptive arm of the immune system, including TH17 cells, which can lead to exacerbation of NAFLD. Additionally, IL-17A stimulates secretion of TGFβ by Kupffer cells, which drives stellate cell activation and release of ECM from fibroblasts. Kupffer cell-derived ROS and ATP also contribute to NAFLD- and NASH-associated inflammation. NAFLD, non-alcoholic fatty liver disease; DAMPs, damage-associated molecular patterns; TH17, T helper 17 cell; IL-17A, TH17-derived interleukin 17A; ECM, extracellular matrix; ROS, reactive oxygen species; NASH, non-alcoholic steatohepatitis.
2. Immunity and NAFLD/NASH
Approximately 30% of patients with NAFLD develop an inflammatory phenotype and progress to NASH, with subsequent tissue injury and the development of hepatic fibrosis. However, this key step between the relatively benign NAFLD phenotype and inflammatory NASH remains subject to extensive debate. Chronic metabolic inflammation (metaflammation) is initially promoted during metabolic diseases such as obesity and type 2 diabetes (T2D), and there is evidence to suggest that this fuels the NAFLD–NASH transition. In particular, the adipose tissue has been identified as a major source of inflammatory cytokines, including pro-inflammatory tumor necrosis factor (TNF), interleukin-1β (IL-1β) and IL-6 [3]. In mouse models of hepatic steatosis, systemic deletion of Tnf and chemical inhibition of TNF-Receptor-1 reduces the prevalence of steatosis and hepatocellular injury [4][5]. At the hepatic level, lipotoxicity within hepatocytes drives the release of CXCL10 (C-X-C motif ligand 10) [6], which is a chemoattractant, or CXCR3 (C-X-C motif chemokine receptor 3)-expressing Kupffer cells [7], the tissue-resident macrophages of the liver. Upon localization to the site of injury and stimulation of toll-like receptor 4 (TLR4), Kupffer cells also release TNF, IL-1β and IL-6 [8]. TNF-alpha (TNFα) is a major contributor to the inflammatory response, regulating various aspects of sickness behavior during infection, including fever and cachexia. In the context of NAFLD, TNFα was shown to drive an increase in the expression of the genes Acaca (acetyl-CoA carboxylase alpha) and Scd1 (stearoyl-CoA desaturase 1) [4]. Acaca encodes a rate-limiting enzyme for fatty acid synthesis [9], whilst Scd1 encodes a lipogenic enzyme that catalyzes the synthesis of monounsaturated fatty acids [10]. Furthermore, inhibition of the TNFα receptor, TNFR1, improves liver steatosis and insulin resistance [5]. Given that TNFα is a major driver of cachexia during infection, it is paradoxical that it also drives increased expression of these two lipid storage enzymes in NAFLD, suggesting that the role of this cytokine is tissue- and context-dependent.
IL-6 is an important driver of adaptive immune recruitment, selectively controlling T cell recruitment by mediating chemokine secretion [11]. Further to recruitment of T cells, IL-6 also drives polarization of CD4+ T cells, inhibiting T helper 1 (TH1) and promoting TH2 and TH17 differentiation [12][13]. In both NAFLD and NASH, patients exhibit increased circulating IFNγ-producing TH1 cells, and patients with NASH could be stratified from those with NAFLD by the increase in circulating TH17 cells [14]. TH17 cells are CD4+ cells that express the transcription factor RORγt (retinoid orphan receptor gamma t) and RORα, and are characterized by secretion of IL-17A and IL-17F. It was recently demonstrated that IL-17A and IL-17F are drivers of adipocyte lipid usage in adipocytes during infection and, moreover, promote infection-induced cachexia [15], suggesting that it may drive lipid usage in other cell types, including hepatocytes. As with TNFα, this appears paradoxical, as NAFLD is associated with increased hepatic lipid storage. However, increased expression of TNFα and IL-17A may represent a mechanism by which the liver attempts to mobilize and dispose of excess lipids to restore homeostatic function.
TH17 cells are known to expand in the liver of obese humans and mice [16], and multiple rodent models of NAFLD show increased IL-17A signaling through the IL-17A receptor (IL-17RA) [17][18]. The development of NAFLD leads to increased infiltration of non-conventional CXCR3+ TH17 cells, which can co-express IFNγ. Adoptive transfer of CXCR3+ TH17 cells into mice with experimental NAFLD increased hepatic damage compared with those given CXCR3− TH17 cells [19]. Furthermore, the livers of mice given CXCR3+ TH17 cells displayed increased triglyceride accumulation and hepatocyte ballooning. Moreover, the presence of CXCR3+ TH17 cells correlated with increased disease severity in humans, suggesting that this is an evolutionarily conserved aspect of NAFLD and NASH. However, the mechanisms by which these CXCR3+ TH17 cells exacerbate disease remain unclear. Typically, TH17 cell-secreted IL-17A recruits and activates neutrophils [20], a cell type abundant in the liver of NASH patients, but it remains to be seen whether CXCR3+ TH17 cells exert their effects through neutrophil activation or an as yet unknown mechanism. Further to its role in recruitment of neutrophils, IL-17A signals through IL-17RA on Kupffer cells to promote production of TGF-β1 (transforming growth factor β1) [21], which, in turn, promotes hepatic stellate cell (HSC) activation and extracellular matrix secretion, contributing to NAFLD progression [22].
3. Immunometabolism in NAFLD/NASH
An emerging area of interest in NAFLD and NASH is immunometabolism. All cells rely on nutrients to function and immune cells are no exception. Indeed, during infection and injury, the immune system requires significant amounts of energy to fuel itself, with different cell types relying on specific nutrients for optimal function. Activation of Kupffer cells leads to enhanced glucose utilization [23] and a rapid increase in aerobic glycolysis (the “Warburg Effect”), whereby cells preferentially rely on glycolysis despite the presence of oxygen [24]. Although glycolysis is less energetically favorable than oxidative phosphorylation (OXPHOS) [25], it carries a number of advantages for immune cells. Early branching of the glycolytic pathway generates precursors for the pentose phosphate pathway (PPP) and de novo nucleotide synthesis, which are required for the function of multiple immune cell types [26]. For example, in macrophages, such as Kupffer cells, the PPP is required to sustain superoxide anion production [27], a key component for the phagocytic oxidative burst [28], which is a tool for destroying pathogens. In the context of NAFLD, a wide variety of cells increase superoxide production, including the aforementioned Kupffer cells, as well as neutrophils and other granulocytes [29], which may contribute to NAFLD-associated oxidative stress. Furthermore, NAFLD is associated with increased aerobic glycolysis [30], leading to increased lactate production and stabilization of HIF-1α (hypoxia-inducible factor 1α). This HIF-1α stabilization, in turn, promotes usage of the glycolytic pathway and promotes liver fibrosis in murine models of NAFLD [31], potentially signaling a key step in the transition from NASH to cirrhosis.
A further aspect of the immune system associated with NAFLD and progression to NASH is the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome. The NLRP3 inflammasome is an intracellular structure that senses microbial compounds and environmental stress. Its assembly leads to production of IL-1β and IL-18, as well as caspase-1-dependent apoptosis [32]. In mouse models of NASH, administration of a selective NLRP3 inhibitor suppressed caspase-1 and IL-1β accumulation, and limited the development of fibrosis [33], suggesting that the inflammasome plays a key role in mediating the progression of NAFLD. One of the major drivers of the inflammasome is ROS (reactive oxygen species) production, generated by OXPHOS. The mitochondrial electron transport chain (ETC) is an essential structure for ATP generation and is composed of multiple complexes (complexes I, II, III, IV and ATPase). Complex II, also known as succinate dehydrogenase, links the ETC with the TCA cycle, and chemical inhibition of this subunit prevents NLRP3 inflammasome activation [34]. Whilst there is evidence to suggest that ROS production leads to activation of the NLRP3 inflammasome [35], recent work suggests that it is ATP generation, and not ROS, that is required for inflammasome activation [34]. Since NAFLD and NASH are prevalent in patients with concurrent obesity and metabolic dysfunction, increased metabolic cycling through glycolysis and OXPHOS may exacerbate inflammasome activation and oxidative damage.
Understanding the immune response mechanism in NAFLD is crucial for developing a complete picture of sexual dimorphism in liver disease. This gap in our knowledge needs addressing as it has critical implications for the development of new therapeutics.
References
- Feldstein, A.E.; Canbay, A.; Angulo, P.; Taniai, M.; Burgart, L.J.; Lindor, K.D.; Gores, G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003, 125, 437–443.
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342.
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose Tissue-Liver Cross Talk in the Control of Whole-Body Metabolism: Implications in Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1899–1912.
- Kakino, S.; Ohki, T.; Nakayama, H.; Yuan, X.; Otabe, S.; Hashinaga, T.; Wada, N.; Kurita, Y.; Tanaka, K.; Hara, K.; et al. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm. Metab. Res. 2017, 50, 80–87.
- Wandrer, F.; Liebig, S.; Marhenke, S.; Vogel, A.; John, K.; Manns, M.P.; Teufel, A.; Itzel, T.; Longerich, T.; Maier, O.; et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020, 11, 1–9.
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2016, 63, 731–744.
- García-López, M.; Sánchez-Madrid, F.; Rodríguez-Frade, J.M.; Mellado, M.; Acevedo, A.; García, M.I.; Albar, J.P.; Martínez-A, C.; Marazuela, M. CXCR3 Chemokine Receptor Distribution in Normal and Inflamed Tissues: Expression on Activated Lymphocytes, Endothelial Cells, and Dendritic Cells. Lab. Investig. 2001, 81, 409–418.
- Fisher, J.E.; McKenzie, T.J.; Lillegard, J.B.; Yu, Y.; Juskewitch, J.E.; Nedredal, G.I.; Brunn, G.J.; Yi, E.S.; Malhi, H.; Smyrk, T.C.; et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J. Surg. Res. 2012, 180, 147–155.
- Kim, K.-H. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 1997, 17, 77–99.
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486.
- McLoughlin, R.M.; Jenkins, B.J.; Grail, D.; Williams, A.S.; Fielding, C.A.; Parker, C.R.; Ernst, M.; Topley, N.; Jones, S.A. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 9589–9594.
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536.
- Harbour, S.N.; DiToro, D.F.; Witte, S.J.; Zindl, C.L.; Gao, M.; Schoeb, T.R.; Jones, G.W.; Jones, S.A.; Hatton, R.D.; Weaver, C.T. T H 17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020, 5, eaaw2262.
- Rau, M.; Schilling, A.-K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105.
- Sinton, M.C.; Girard, A.; Ogunsola, J.; Chandrasegaran, P.; Capewell, P.; Perona-Wright, G.; Quintana, J.F. Interleukin-17 drives sex-dependent weight loss and changes in feeding behaviour during Trypanosoma brucei infection. bioRxiv 2022.
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.C.; Lawson, M.J.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838.
- Zúñiga, L.A.; Shen, W.-J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol. 2010, 185, 6947–6959.
- Harley, I.T.; Stankiewicz, T.E.; Giles, D.A.; Softic, S.; Flick, L.M.; Cappelletti, M.; Sheridan, R.; Xanthakos, S.A.; Steinbrecher, K.A.; Sartor, R.B.; et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 2013, 59, 1830–1839.
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.S.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab. 2021, 33, 1187–1204.e9.
- González-Terán, B.; Matesanz, N.; Nikolic, I.; Verdugo, M.A.; Sreeramkumar, V.; Hernández-Cosido, L.; Sabio, G. p38γ and p38δ reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 2016, 35, 536–552.
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3.
- Nair, B.; Nath, L.R. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J. Recept. Signal Transduct. 2020, 40, 195–200.
- Meszaros, K.; Bojta, J.; Bautista, A.P.; Lang, C.; Spitzer, J.J. Glucose utilization by Kupffer cells, endothelial cells, and granulocytes in endotoxemic rat liver. Am. J. Physiol. Liver Physiol. 1991, 260, G7–G12.
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530.
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.-P. Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions. Front. Immunol. 2020, 11, 202.
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643.
- Spolarics, Z.; Bautista, A.; Spitzer, J. Primed pentose cycle activity supports production and elimination of superoxide anion in Kupffer cells from rats treated with endotoxin in vivo. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1993, 1179, 134–140.
- Luo, B.; Wang, J.; Liu, Z.; Shen, Z.; Shi, R.; Liu, Y.-Q.; Jiang, M.; Wu, Y.; Zhang, Z. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat. Commun. 2016, 7, 12177.
- El-Benna, J.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A. Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008, 30, 279–289.
- Li, J.; Wang, T.; Xia, J.; Yao, W.; Huang, F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. FASEB J. 2019, 33, 11640–11654.
- Mesarwi, O.A.; Moya, E.A.; Zhen, X.; Gautane, M.; Zhao, H.; Giró, P.W.; Alshebli, M.; McCarley, K.E.; Breen, E.C.; Malhotra, A. Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease. Am. J. Respir. Cell Mol. Biol. 2021, 65, 390–402.
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489.
- Mridha, A.R.; Wree, A.; Robertson, A.A.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.-H.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046.
- Billingham, L.K.; Stoolman, J.S.; Vasan, K.; Rodriguez, A.E.; Poor, T.A.; Szibor, M.; Jacobs, H.T.; Reczek, C.R.; Rashidi, A.; Zhang, P.; et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 2022, 23, 692–704.
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225.
More
Information
Subjects:
Cell & Tissue Engineering; Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
850
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




