
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nor Nadiah Abdul Karim Shah | -- | 4665 | 2023-06-11 14:48:48 | | | |
| 2 | Camila Xu | Meta information modification | 4665 | 2023-06-12 04:29:46 | | |
Video Upload Options
Milk processing can cause the demolition of the milk fat globule membrane and induce interactions between whey protein and casein with membranes, leading to changes in pH, protein, and lactose content, as well as the destruction of vitamins and enzymes, hydrolysis of proteins and lipids, disruption of calcium and phosphorus equilibrium, and reduction of the cream layer. Pulsed electric fields (PEF) are gaining recognition in food processing due to their energy efficiency, minimal energy loss, flexibility, instantaneity, non-thermal nature, and environmental friendliness. It has also been found to reduce spoilage by microorganisms and the inactivation of undesirable enzymes, as well as its better retention of organoleptic and nutritional characteristics.
1. Introduction
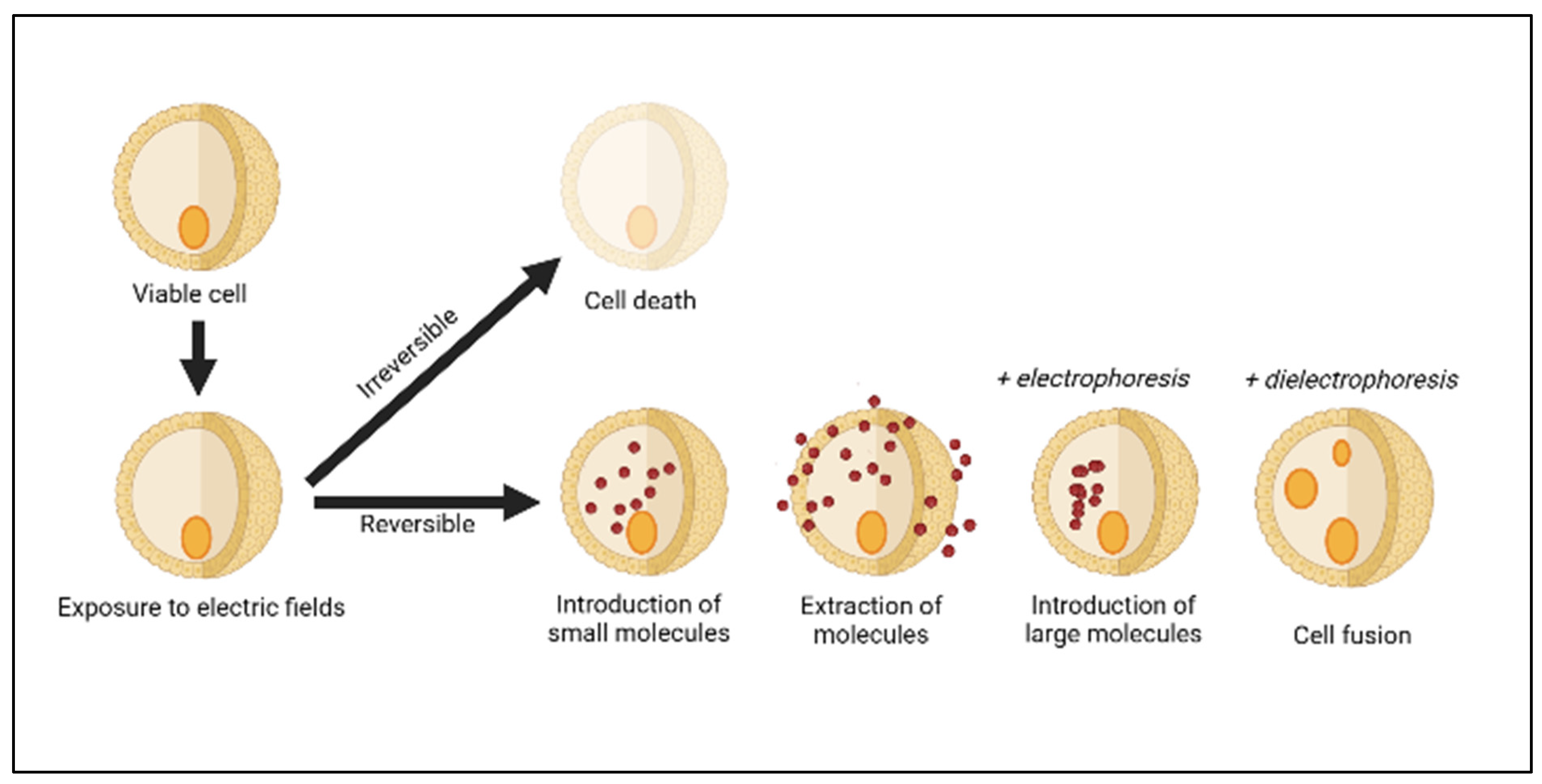
2. Pulsed Electric Fields System
3. Effect of Pulsed Electric Fields on Milk Nutrients
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Fats and fatty acids | Raw bovine milk and milk with different MFG sizes | 16 kV/cm; 30 µs; 100 mL/min; 25 °C | Increase in FAs and some unknown long-chain FAs in raw milk and milk with large MFGs; a slight decrease in the fat content | [19] |
| Fatty acid profiles | Raw goat milk | 20, 30, and 40 kV/cm; 5 and 10 μs; 2.5 L/h; 30 °C | Reduction in the total SFAs and total PUFAs; increase in total MUFAs | [30] |
| Fatty acids | Fruit juices-milk beverages | 35 kV/cm; 1800 μs; 60 mL/min; 60 mL/s; <40 °C | 20% increase in linoleic acid; decrease in palmitic, linoleic, and linolenic acids (12–20%) at day 56 | [31] |
| Fatty acid profiles | Raw whole bovine milk (4% fat) | 30 kV/cm; 22 μs; 2.4 L/min; 53 and 63 °C | No significant changes (p > 0.05) in fat content; minor increase in butanoic (C4:0), hexanoic (C6:0), and octanoic (C8:0) | [22] |
| Fats | Bovine whole milk | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 55 °C | Increase fat melting from a-crystal into b-crystal conversion | [20] |
| MFG | Bovine whole milk (44% fat) | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 17–22 °C | Reduction in MFGs’ size; increase in ζ-potential and specific surface area; plasma protein absorption on MFGM surface | [21] |
| Milk fat | Reconstituted skimmed milk | 15–20 kV/cm; 20–60 pulses; <35 °C | Increase the surface area of MFGs | [27] |
| Volatile compounds (including fatty acids) | Raw bovine milk | 15–30 kV/cm; 800 µs; <40 °C | No significant changes (p > 0.05) in methyl ketones [saturated fatty acids); no changes in concentration of short-chain fatty acid | [29] |
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Proteins | Raw bovine milk and milk with different MFG sizes | 16 kV/cm; 30 µs; 100 mL/min; 25 °C | 15% and 29% decrease in β-Lg and α-La respectively (in milk with small MFGs); more small protein aggregates (<40 kDa) appeared | [19] |
| Native proteins | 10% solution of liquid whey protein | 50 kV/cm; 3–8 µs; 5 L/h; 20–40 °C | Stable IgM and IgG | [48] |
| Whey proteins | Bovine whole milk | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 55 °C | Increase in the hydrophobic surface of the protein; reducion in milk protein denaturation | [20] |
| Caseins and whey proteins | Reconstituted skim milk (10 wt%) | 49 kV/cm; 19.36 µs; 240 mL/min; 20–72 °C | Increase in the amount of protein in the serum; increase the number of caseins in serum; no changes in whey proteins | [23] |
| Protein compositions | Fresh raw cream and pasteurized cream (40% fat) | 37 kV/cm; 1705 μs; 25 mL/min; 50 and 65 °C |
No changes in phospholipids; induced interactions of β-Lg and MFGM proteins (at 65 °C). |
[49] |
| Milk proteins | Raw whole milk | 40 kV/cm; <15 s; 30 L/h; 72 °C | 70% decrease in the native form of milk proteins including IgG, IgA, and lactoferrin | [24] |
| Protein | BSA | 20–35 kV/cm; 400 μs; 200 Hz; <30 °C | No self-aggregation of BSA; no significant difference (p > 0.05) in molecular weight distribution profiles of BSA | [50] |
| Protein content | Raw skim and raw whole milk | 30.76–53.84 kV/cm; 12–30 pulses, 1 L/min; 20–40 °C | A 0.17% decrease in protein content | [26] |
| Whey protein | Whey protein isolate | 12–20 kV/cm; 10–20 pulses; <35 °C | Increase polarity and unfolding whey proteins; partial denaturation of whey fractions | [51] |
| Protein aggregation | Whey protein isolate | 30–35 kV/cm; 19.2–211 µs; 60 mL/min; 30–75 °C | No changes in protein aggregation and surface hydrophobicity; partial denaturation of native lactoferrin, apo-lactoferrin, and halo-lactoferrin | [52] |
| Milk casein micelles’ sizes | Raw milk, reconstituted skim milk, concentrated skim milk, and milk protein concentrates | 45 kV/cm; 20 µs; 240 mL/min; 30 °C | Decrease in casein micelles’ sizes | [28] |
| Proteolysis profiles (peptide and free amino acid concentration) | Raw milk cheese | 30 kV/cm; 80–120 pulses; 6 mL/min; 50 °C). | Intermediate proteolysis profiles between raw milk and thermally pasteurized milk Severe enzyme modification reduces the formation of peptides and amino acids at longer pulses |
[53] |
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Vitamin A (as β-carotene) and B-group vitamins (thiamine, riboflavin, and niacin) | Raw goat milk | 20, 30, and 40 kV/cm; 5–13 µs; 2.5 L/min; 30 °C | >94% retention of vitamin A (as β-carotene); 95–99% retention of B-group vitamins | [12] |
| Vitamins A and C | 10% solution of liquid whey protein | 50 kV/cm; 3–8 µs; 5 L/h; 20–40 °C | No changes in vitamins A and C | [48] |
| Total carotenoids (provitamin A), vitamin C, in vitro bioaccessibility of vitamin C | Fruit juice and milk beverages | 35 kV/cm; 1800 µs; 60 mL/min; <35 °C | 15% increase in the bioaccessibility of total carotenoids (provitamin A); 8–15% reduction of vitamin C; no changes in bio-accessibility of vitamin C | [32] |
| Total carotenoids (provitamin A), vitamin C (ascorbic acid) | Orange juice-milk beverage | 25 kV/cm; 280 µs; 60 mL/min; 57 °C | 2% destruction of ascorbic acid;5% destruction of total carotenoids | [33] |
| B-group vitamins (niacin, thiamin, and riboflavin), vitamin C | FJ-WM and FJ-SM beverages | 35 kV/cm; 1800 µs; 760 mL/min; <40 °C | 95–99.8% retention of group B vitamins (niacin, thiamin, and riboflavin); 97% retention of vitamin C in FJ-WM; 99.5% retention of vitamin C in FJ-SM | [34] |
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Fe, Cr, Ni, and Mn | Raw goat milk | 20, 30, and 40 kV/cm; 5 and 10 μs; 2.5 L/h; 30 °C | Significant increase (p < 0.05) in Fe; A non-significant increase in (p > 0.05) in Cr, Ni, and Mn |
[30] |
| Fe, Cr, and Ni | 2% partially skimmed milk | 30–40 kV/cm; 500 mL/min; 20–25 °C | A significant increase (p < 0.05) in Fe | [25] |
3.1. Fats and Fatty Acids
3.2. Proteins and Amino Acids
3.3. Vitamins
3.4. Minerals
References
- Altomonte, I.; Salari, F.; Martini, M. Milk fat components and milk quality. In Microbiology in Dairy Processing: Challenges and Opportunities; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 8, pp. 1–10.
- Yang, S.; Liu, G.; Qin, Z.; Munk, D.; Otte, J.; Ahrne, L. Effect of Emerging Processing Methods on the Food Quality: Advantages and Challenges; Springer: Berlin/Heidelberg, Germany, 2019; pp. 27–67.
- O’Callaghan, T. The benefits of pasture-based dairy. Ir. Food 2019, 1, 34–35.
- Zheroz. Importance of Micronutrients and Macronutrients in Your Daily Diet. Available online: https://zheroz.wordpress.com/2018/09/19/importance-of-micronutrients-and-macronutrients-in-your-daily-diet/ (accessed on 1 September 2022).
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P., Jr.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289.
- Lu, C.D.; Miller, B.A. Current status, challenges and prospects for dairy goat production in the Americas. Asian-Australas. J. Anim. Sci. 2019, 32, 1244–1255.
- Sakkas, L.; Moutafi, A.; Moschopoulou, E.; Moatsou, G. Assessment of heat treatment of various types of milk. Food Chem. 2014, 159, 293–301.
- Khanam, T.G.; Goswami, M.; Pathak, V.; Bharti, S.K.; Karunakara, K.N. Pulse electric field: Novel approach to dairy industry. Indian Food Ind. Mag. 2017, 36, 8–12.
- Najib, M.; Hallab, M.W.; Hallab, K.; Hallab, Z.; Hamze, M.; Chihib, N. Thermal processing of milk as a main tool in the production of Qishta, Khoa and Kajmak. J. Mater. Environ. Sci. 2020, 11, 294–309.
- Borad, S.G.; Kumar, A.; Singh, A.K. Effect of processing on nutritive values of milk protein. Crit. Rev. Food Sci. Nutr. 2017, 57, 3690–3702.
- Sharma, P.; Oey, I.; Bremer, P.; Everett, D.W. Microbiological and enzymatic activity of bovine whole milk treated by pulsed electric fields. Int. J. Dairy Technol. 2017, 71, 10–19.
- Mohamad, A.; Shah, N.N.A.K.; Sulaiman, A.; Mohd Adzahan, N.; Aadil, R.M. Impact of the pulsed electric field on physicochemical properties, fatty acid profiling, and metal migration of goat milk. J. Food Process. Preserv. 2021, 44, e14940.
- Scudino, H.; Silva, E.K.; Gomes, A.; Guimarães, J.T.; Cunha, R.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Ultrasound stabilization of raw milk: Microbial and enzymatic inactivation, physicochemical properties and kinetic stability. Ultrason. Sonochemistry 2020, 67, 105185.
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; El-Din Bekhit, A.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13.
- Pal, M. Pulsed Electric Field Processing: An Emerging Technology for Food Preservation. J. Exp. Food Chem. 2017, 3, 1000126.
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411.
- Ahmad, T.; Butt, M.Z.; Aadil, R.M.; Inam-Ur-Raheem, M.; Bekhit, A.E.D.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q. Impact of nonthermal processing on different milk enzymes. Int. J. Dairy Technol. 2019, 72, 481–495.
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.D. The impact of nonthermal technologies on the microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457.
- Yang, S.; Suwal, S.; Andersen, U.; Otte, J.; Ahrné, L. Effects of pulsed electric field on fat globule structure, lipase activity, and fatty acid composition in raw milk and milk with different fat globule sizes. Innov. Food Sci. Emerg. Technol. 2021, 67, 102548.
- Sharma, P.; Oey, I.; Everett, D.W. Thermal properties of milk fat, xanthine oxidase, caseins and whey proteins in pulsed electric field-treated bovine whole milk. Food Chem. 2016, 207, 34–42.
- Sharma, P.; Oey, I.; Everett, D.W. Interfacial properties and transmission electron microscopy revealing damage to the milk fat globule system after pulsed electric field treatment. Food Hydrocoll. 2015, 47, 99–107.
- McAuley, C.M.; Singh, T.K.; Haro-Maza, J.F.; Williams, R.; Buckow, R. Microbiological and physicochemical stability of raw, pasteurised or pulsed electric field-treated milk. Innov. Food Sci. Emerg. Technol. 2016, 38, 365–373.
- Liu, Z.; Hemar, Y.; Tan, S.; Sanguansri, P.; Niere, J.; Buckow, R.; Augustin, M.A. Pulsed electric field treatment of reconstituted skim milks at alkaline pH or with added EDTA. J. Food Eng. 2015, 144, 112–118.
- Mathys, A.; Toepfl, S.; Siemer, C.; Favre, L.; Benyacoub, J.; Hansen, C.E. Pulsed Electric Field Treatment Process and Dairy Product Comprising Bioactive Molecules Obtainable by the. Process. Patent No WO2013007620A1, 17 January 2013.
- Gad, A.; Jayaram, S.H. Effect of electric pulse parameters on releasing metallic particles from stainless steel electrodes during PEF processing of milk. IEEE Trans. Ind. Appl. 2013, 50, 1402–1409.
- Bermúdez-Aguirre, D.; Fernández, S.; Esquivel, H.; Dunne, P.C.; Barbosa-Cánovas, G.V. Milk processed by pulsed electric fields: Evaluation of microbial quality, physicochemical characteristics, and selected nutrients at different storage conditions. J. Food Sci. 2011, 76, S289–S299.
- Xiang, B.; Simpson, M.; Ngadi, M.; Simpson, B. Flow behaviour and viscosity of reconstituted skimmed milk treated with pulsed electric field. Biosyst. Eng. 2011, 109, 228–234.
- Hemar, Y.; Augustin, M.A.; Cheng, L.J.; Sanguansri, P.; Swiergon, P.; Wan, J. The effect of pulsed electric field processing on particle size and viscosity of milk and milk concentrates. Milchwiss.-Milk Sci. Int. 2011, 66, 126.
- Zhang, S.; Yang, R.; Zhao, W.; Hua, X.; Zhang, W.; Zhang, Z. Influence of pulsed electric field treatments on the volatile compounds of milk in comparison with pasteurized processing. J. Food Sci. 2011, 76, C127–C132.
- Mohamad, A.; Abdul Karim Shah, N.N.; Sulaiman, A.; Mohd Adzahan, N.; Aadil, R.M. Pulsed electric field of goat milk: Impact on Escherichia coli ATCC 8739 and vitamin constituents. J. Food Process Eng. 2021, 44, e13779.
- Salvia-Trujillo, L.; Morales-de la Pena, M.; Rojas-Grau, A.; Welti-Chanes, J.; Martin-Belloso, O. Mineral and fatty acid profile of high intensity pulsed electric fields or thermally treated fruit juice-milk beverages stored under refrigeration. Food Control 2017, 80, 236–243.
- Rodriguez-Roque, M.J.; de Ancos, B.; Sanchez-Vega, R.; Sanchez-Moreno, C.; Cano, M.P.; Elez-Martineza, P.; Martin-Belloso, O. Food matrix and processing influence on carotenoid bioaccessibility and lipophilic antioxidant activity of fruit juice-based beverages. Food Funct. 2016, 1, 380–389.
- Zulueta, A.; Barba, F.J.; Esteve, M.J.; Frigola, A. Changes in quality and nutritional parameters during refrigerated storage of orange juice-milk beverage treated by equivalent thermal and non-thermal processes for mild pasteurization. Food Bioprocess Technol. 2013, 6, 2018–2030.
- Salvia-Trujillo, L.; Morales-de la Pena, M.; Rojas-Grau, A.; Martin-Belloso, O. Changes in water-soluble vitamins and antioxidant capacity of fruit juice-milk beverages as affected by high-intensity pulsed electric fields (HIPEF) or heat during chilled storage. J. Agric. Food Chem. 2011, 59, 10034–10043.
- Gómez, B.; Munekata, P.E.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105.
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377.
- Manzoor, M.F.; Zeng, X.A.; Ahmad, N.; Ahmed, Z.; Rehman, A.; Aadil, R.M.; Roobab, U.; Siddique, R. Effect of pulsed electric field and thermal treatments on the bioactive compounds, enzymes, microbial and physical stability of almond milk during storage. J. Food Process. Preser. 2020, 44, e14541.
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chemical engineering and processing. Process Intensif. 2007, 46, 537–546.
- Elez-Martínez, P.; Odriozola-Serrano, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of pulsed electric fields processing strategies on health-related compounds of plant-based foods. Food Eng. Rev. 2017, 9, 213–225.
- Syed, Q.A.; Ishaq, A.; Rahman, U.U.; Aslam, S.; Shukat, R. Pulsed electric field technology in food preservation: A review. J. Nutr. Health Food Eng. 2017, 6, 168–172.
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Ahmad, M.H.; Malik, N.; Bekhit, A.E.D.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54.
- Ozturk, B.; Anli, E. Pulsed electric fields (PEF) applications on wine production: A review. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2017; p. 02008.
- Liu, Z.; Zhao, L.; Zhang, Q.; Huo, N.; Shi, X.; Li, L.; Jia, L.; Lu, Y.; Peng, Y.; Song, Y. Proteomics-based mechanistic investigation of Escherichia coli inactivation by pulsed electric field. Front. Microbiol. 2019, 10, 2644.
- Montanari, C.; Tylewicz, U.; Tabanelli, G.; Berardinelli, A.; Rocculi, P.; Ragni, L.; Gardini, F. Heat-assisted pulsed electric field treatment for the inactivation of Saccharomyces cerevisiae: Effects of the presence of citral. Front. Microbiol. 2019, 10, 1737.
- Alahakoon, A.U.; Oey, I.; Bremer, P.; Silcock, P. Process optimisation of pulsed electric fields pre-treatment to reduce the sous vide processing time of beef briskets. Int. J. Food Sci. Technol. 2019, 54, 823–834.
- Zhu, N.; Zhu, Y.; Yu, N.; Wei, Y.; Zhang, J.; Hou, Y.; Sun, A.-d. Evaluation of microbial, physicochemical parameters and flavor of blueberry juice after microchip-pulsed electric field. Food Chem. 2019, 274, 146–155.
- Mannozzi, C.; Rompoonpol, K.; Fauster, T.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Influence of pulsed electric field and ohmic heating pretreatments on enzyme and antioxidant activity of fruit and vegetable juices. Foods 2019, 8, 247.
- Schottroff, F.; Gratz, M.; Krottenthaler, A.; Johnson, N.B.; Bedard, M.F.; Jaeger, H. Pulsed electric field preservation of liquid whey protein formulations–influence of process parameters, pH, and protein content on the inactivation of Listeria innocua and the retention of bioactive ingredients. J. Food Eng. 2019, 243, 142–152.
- Xu, S.; Walkling-Ribeiro, M.; Griffiths, M.; Corredig, M. Pulsed electric field processing preserves the antiproliferative activity of the milk fat globule membrane on colon carcinoma cells. J. Dairy Sci. 2015, 98, 2867–2874.
- Zhao, W.; Yang, R. Pulsed electric field induced aggregation of food proteins: Ovalbumin and bovine serum albumin. Food Bioprocess Technol. 2012, 5, 1706–1714.
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed electric field-induced structural modification of whey protein isolate. Food Bioprocess Technol. 2011, 4, 1341–1348.
- Sui, Q.; Roginski, H.; Williams, R.P.; Versteeg, C.; Wan, J. Effect of pulsed electric field and thermal treatment on the physicochemical and functional properties of whey protein isolate. Int. Dairy J. 2011, 21, 206–213.
- Yu, L.J.; Ngadi, M.; Raghavan, V. Proteolysis of cheese slurry made from pulsed electric field-treated milk. Food Bioprocess Technol. 2012, 5, 47–54.
- Banjare, K.; Kumar, M.; Kumar, R.; Kartikyen, S.; Goel, B.; Uprit, S. Perspective role of goat milk and products: A review. Int. J. Chem. Stud. 2017, 5, 1328–1338.
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of lipids in foods. Sarhad J. Agric. 2016, 32, 230–238.
- Salari, S.; Jafari, S.M. The influence of ohmic heating on degradation of food bioactive ingredients. Food Eng. Rev. 2020, 12, 191–208.
- Ghnimi, S.; Budilarto, E.; Kamal-Eldin, A. The new paradigm for lipid oxidation and insights to microencapsulation of omega-3 fatty acids. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1206–1218.
- Deeth, H.; Fitz-Gerald, C. Lipolytic enzymes and hydrolytic rancidity. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2006; Volume 2, pp. 481–556.
- Shinn, S.E.; Ruan, C.M.; Proctor, A. Strategies for producing and incorporating conjugated linoleic acid–rich oils in foods. Annu. Rev. Food Sci. Technol. 2017, 8, 181–204.
- Garcia-Amezquita, L.; Primo-Mora, A.; Barbosa-Cánovas, G.; Sepulveda, D. Effect of nonthermal technologies on the native size distribution of fat globules in bovine cheese-making milk. Innov. Food Sci. Emerg. Technol. 2009, 10, 491–494.
- Pataro, G.; Ferrari, G. Limitations of pulsed electric field utilization in food industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310.
- Ranadheera, C.; Evans, C.; Baines, S.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.; Naumovski, N. Probiotics in goat milk products: Delivery capacity and ability to improve sensory attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882.
- Turkmen, N.; Akal, C.; Özer, B. Probiotic dairy-based beverages: A review. J. Funct. Foods 2019, 53, 62–75.
- O’mahony, J.A.; Fox, P.F. Milk Proteins: Introduction and Historical Aspects. In Advanced Dairy Chemistry Volume 2 Lipids, 4th ed.; McSweeney, P.L., Fox, P.F., Eds.; Springer Science & Business Media: Boston, MA, USA, 2013; Volume 1A, pp. 43–85.
- Duque, S.M.M.; Leong, S.Y.; Agyei, D.; Singh, J.; Larsen, N.; Oey, I. Understanding the impact of Pulsed Electric Fields treatment on the thermal and pasting properties of raw and thermally processed oat flours. Food Res. Int. 2020, 129, 108839.
- Zhang, Z.-H.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z.; Xiong, X.-Y. Effects of pulsed electric fields (PEF) on vitamin C and its antioxidant properties. Int. J. Mol. Sci. 2015, 16, 24159–24173.
- Buckow, R.; Chandry, P.S.; Ng, S.Y.; McAuley, C.M.; Swanson, B.G. Opportunities and challenges in pulsed electric field processing of dairy products. Int. Dairy J. 2014, 34, 199–212.
- Yilmaz, M.; Evrendilek, G.A. Impact of the pulsed electric field treatment on bioactive food compounds: Bioaccessibility and bioavailability. Food Sci. Nutr. 2017, 7, 1–6.
- Sharma, P.; Oey, I.; Everett, D.W. Effect of pulsed electric field processing on the functional properties of bovine milk. Trends Food Sci. Technol. 2014, 35, 87–101.
- Michalski, M.-C.; Michel, F.; Sainmont, D.; Briard, V. Apparent ζ-potential as a tool to assess mechanical damages to the milk fat globule membrane. Colloids Surf. B Biointerfaces 2002, 23, 23–30.
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Effect of high intensity pulsed electric fields on enzymes and vitamins in bovine raw milk. Int. J. Dairy Technol. 2009, 62, 1–6.
- Bendicho, S.; Espachs, A.; Arantegui, J.; Martín, O. Effect of high intensity pulsed electric fields and heat treatments on vitamins of milk. J. Dairy Res. 2002, 69, 113–123.
- Noël, L.; Chekri, R.; Millour, S.; Vastel, C.; Kadar, A.; Sirot, V.; Leblanc, J.-C.; Guérin, T. Li, Cr, Mn, Co, Ni, Cu, Zn, Se and Mo levels in foodstuffs from the Second French TDS. Food Chem. 2012, 132, 1502–1513.
- Holt, C. Encyclopedia of Dairy Sciences; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 917–924.
- Poulsen, N.A.; Rybicka, I.; Poulsen, H.D.; Larsen, L.B.; Andersen, K.K.; Larsen, M.K. Seasonal variation in content of riboflavin and major minerals in bulk milk from three Danish dairies. Int. Dairy J. 2015, 42, 6–11.
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Samaržija, D. Mineral elements in milk and dairy products. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2012, 62, 111–125.




