Milk processing can cause the demolition of the milk fat globule membrane and induce interactions between whey protein and casein with membranes, leading to changes in pH, protein, and lactose content, as well as the destruction of vitamins and enzymes, hydrolysis of proteins and lipids, disruption of calcium and phosphorus equilibrium, and reduction of the cream layer. Pulsed electric fields (PEF) are gaining recognition in food processing due to their energy efficiency, minimal energy loss, flexibility, instantaneity, non-thermal nature, and environmental friendliness. It has also been found to reduce spoilage by microorganisms and the inactivation of undesirable enzymes, as well as its better retention of organoleptic and nutritional characteristics.
- pulsed electric fields
- milk
- macronutrients
- micronutrients
1. Introduction
2. Pulsed Electric Fields System
The PEF system consists of a few components, including a repetitive high-voltage pulse generator, treatment chamber, pump or liquid handling system, and control devices [14][35][14,35]. Depending on the design of the treatment chamber and the type of treated sample, either a continuous or batch chamber can be operated. A continuous chamber is preferred for industrial applications due to the continuous flow of the samples, while a batch or static chamber can only process the given amount of liquid, solid, or semi-solid samples [14]. Nevertheless, both chambers can achieve similar output and comparable levels of microbial and enzymatic inactivation [14][36][37][14,36,37]. In the PEF process, the sample is positioned in the middle of two or more electrodes before being exposed to high-voltage electric field pulses [38]. PEF can operate at a wide range of ambient temperatures, including ambient (20–25 °C), sub-ambient (<20 °C), and above-ambient (<25 °C) temperatures [39][40][39,40], with a short processing time (µs − ms) [18]. PEF can also be applied by varying processing parameters such as electric field strength, pulse width, and pulse frequency, depending on the sample compositions and processing objectives to be acquired [14][18][36][41][14,18,36,41]. Generally, two mechanisms have been suggested for cell inactivation by PEF, namely electrical breakdown and electroporation [18]. Electrical breakdown occurs when the PEF achieves a greater electric field strength than the critical field strength of the microorganism, resulting in direct discharge and the breaking down of the cell membranes [42]. The biological cells are an electrolyte enclosed by an electrically injured membrane, which is exposed to an extrinsic electric field and subsequently induces transmembrane voltage [38]. The possible outcomes of cell electroporation are shown in Figure 1. Electroporation is the process of temporarily weakening the lipid bilayer and proteins of the cell membranes, resulting in the transformation of cell membrane permeability and the subsequent draining of the membrane [43][44][43,44]. It can be reversible or irreversible, depending on the PEF processing parameter, medium constituents, type, and size of the cells [14]. PEF has been used in a variety of food processing applications such as meat processing [45] and the retention of bioactive compounds from fruits and vegetables [46]. Although irreversible, PEF is used for microbial and enzyme inactivation in liquid foods such as milk and fruit juices [30][47][30,47].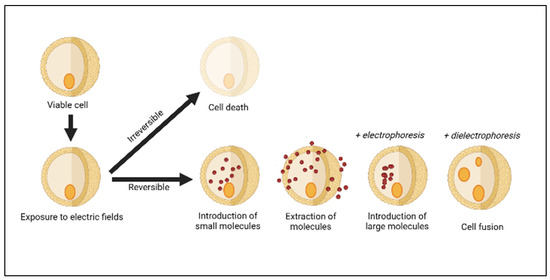
3. Effect of Pulsed Electric Fields on Milk Nutrients
Table 1, Table 2, Table 3 and Table 4 summarize the effects of PEF on fats and fatty acids, proteins and amino acids, vitamins, and minerals in milk, respectively.| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
| Fats and fatty acids | Raw bovine milk and milk with different MFG sizes | 16 kV/cm; 30 µs; 100 mL/min; 25 °C | Increase in FAs and some unknown long-chain FAs in raw milk and milk with large MFGs; a slight decrease in the fat content | [19] |
| ] | ||||
| Nutrient Constituents | Products | PEF Parameter | Effects | References |
|---|---|---|---|---|
| Vitamin A (as β-carotene) and B-group vitamins (thiamine, riboflavin, and niacin) | Raw goat milk | |||
| Fatty acid profiles | Raw goat milk | 20, 30, and 40 kV/cm; 5 and 10 μs; 2.5 L/h; 30 °C | Reduction in the total SFAs and total PUFAs; increase in total MUFAs | [30] |
| Fatty acids | Fruit juices-milk beverages | 35 kV/cm; 1800 μs; 60 mL/min; 60 mL/s; <40 °C | 20% increase in linoleic acid; decrease in palmitic, linoleic, and linolenic acids (12–20%) at day 56 | [31] |
| Volatile compounds (including fatty acids) | ||||
| Raw bovine milk | ||||
| 15–30 kV/cm; 800 µs; <40 °C | No significant changes ( | p | > 0.05) in methyl ketones [saturated fatty acids); no changes in concentration of short-chain fatty acid | [29] |
| Nutrient Constituents | Products | PEF Parameter | Effects | References | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | Raw bovine milk and milk with different MFG sizes | 16 kV/cm; 30 µs; 100 mL/min; 25 °C | 20, 30, and 40 kV/cm; 5–13 µs; 2.5 L/min; 30 °C15% and 29% decrease in β-Lg and α-La respectively (in milk with small MFGs); more small protein aggregates (<40 kDa) appeared | >94% retention of vitamin A (as β-carotene); 95–99% retention of B-group vitamins[19 | [12] | |||||||||||
| Fe, Cr, Ni, and Mn | Raw goat milk | 20, 30, and 40 kV/cm; 5 and 10 μs; 2.5 L/h; 30 °C | Significant increase (p < 0.05) in Fe; A non-significant increase in (p > 0.05) in Cr, Ni, and Mn | ] | ||||||||||||
| [ | 30 | ] | Native proteins | 10% solution of liquid whey protein | 50 kV/cm; 3–8 µs; 5 L/h; 20–40 °C | Stable IgM and IgG | [48] | |||||||||
| Vitamins A and C | 10% solution of liquid whey protein | |||||||||||||||
| Fe, Cr, and Ni | 2% partially skimmed milk | 50 kV/cm; 3–8 µs; 5 L/h; 20–40 °C | No changes in vitamins A and C | [48] | ||||||||||||
| 30–40 kV/cm; 500 mL/min; 20–25 °C | A significant increase ( | p | < 0.05) in Fe | [25] | Whey proteins | Bovine whole milk | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 55 °C | Increase in the hydrophobic surface of the protein; reducion in milk protein denaturation | Total carotenoids (provitamin A), vitamin C, in vitro bioaccessibility of vitamin C[20] | Fruit juice and milk beverages | 35 kV/cm; 1800 µs; 60 mL/min; <35 °C | 15% increase in the bioaccessibility of total carotenoids (provitamin A); 8–15% reduction of vitamin C; no changes in bio-accessibility of vitamin C | [32] | Fatty acid profiles | Raw whole bovine milk (4% fat) | 30 kV/cm; 22 μs; 2.4 L/min; 53 and 63 °C |
| Caseins and whey proteins | Reconstituted skim milk (10 wt%) | No significant changes ( | p > 0.05) in fat content; minor increase in butanoic (C4:0), hexanoic (C6:0), and octanoic (C8:0) | [22] | ||||||||||||
| 49 kV/cm; 19.36 µs; 240 mL/min; 20–72 °C | Increase in the amount of protein in the serum; increase the number of caseins in serum; no changes in whey proteins | [ | 23 | ] | ||||||||||||
| Total carotenoids (provitamin A), vitamin C (ascorbic acid) | Orange juice-milk beverage | 25 kV/cm; 280 µs; 60 mL/min; 57 °C | 2% destruction of ascorbic acid;5% destruction of total carotenoids | [33] | Fats | |||||||||||
| B-group vitamins (niacin, thiamin, and riboflavin), vitamin C | Bovine whole milk | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 55 °C | Increase fat melting from a-crystal into b-crystal conversion | FJ-WM and FJ-SM beverages[20] | ||||||||||||
| 35 kV/cm; 1800 µs; 760 mL/min; <40 °C | 95–99.8% retention of group B vitamins (niacin, thiamin, and riboflavin); 97% retention of vitamin C in FJ-WM; 99.5% retention of vitamin C in FJ-SM | [ | MFG | Bovine whole milk (44% fat) | 20 and 26 kV/cm; 34 μs; 4.2 mL/s; 17–22 °C | Reduction in MFGs’ size; increase in ζ-potential and specific surface area; plasma protein absorption on MFGM surface | ||||||||||
| Protein compositions | Fresh raw cream and pasteurized cream (40% fat) | 37 kV/cm; 1705 μs; 25 mL/min; 50 and 65 °C |
No changes in phospholipids; induced interactions of β-Lg and MFGM proteins (at 65 °C). |
[49] | 34 | [21] | ||||||||||
| Milk proteins | Raw whole milk | 40 kV/cm; <15 s; 30 L/h; 72 °C | 70% decrease in the native form of milk proteins including IgG, IgA, and lactoferrin | [24] | Milk fat | Reconstituted skimmed milk | 15–20 kV/cm; 20–60 pulses; <35 °C | Increase the surface area of MFGs | [27] | |||||||
| Protein | BSA | 20–35 kV/cm; 400 μs; 200 Hz; <30 °C | No self-aggregation of BSA; no significant difference (p > 0.05) in molecular weight distribution profiles of BSA | [50] | ||||||||||||
| Protein content | Raw skim and raw whole milk | 30.76–53.84 kV/cm; 12–30 pulses, 1 L/min; 20–40 °C | A 0.17% decrease in protein content | [26] | ||||||||||||
| Whey protein | Whey protein isolate | 12–20 kV/cm; 10–20 pulses; <35 °C | Increase polarity and unfolding whey proteins; partial denaturation of whey fractions | [51] | ||||||||||||
| Protein aggregation | Whey protein isolate | 30–35 kV/cm; 19.2–211 µs; 60 mL/min; 30–75 °C | No changes in protein aggregation and surface hydrophobicity; partial denaturation of native lactoferrin, apo-lactoferrin, and halo-lactoferrin | [52] | ||||||||||||
| Milk casein micelles’ sizes | Raw milk, reconstituted skim milk, concentrated skim milk, and milk protein concentrates | 45 kV/cm; 20 µs; 240 mL/min; 30 °C | Decrease in casein micelles’ sizes | [28] | ||||||||||||
| Proteolysis profiles (peptide and free amino acid concentration) | Raw milk cheese | 30 kV/cm; 80–120 pulses; 6 mL/min; 50 °C). | Intermediate proteolysis profiles between raw milk and thermally pasteurized milk Severe enzyme modification reduces the formation of peptides and amino acids at longer pulses |
[53] |
3.1. Fats and Fatty Acids
Fats or lipids are the primary elements of milk that influence the cost, nutrition, functional, and sensory attributes of dairy products [54]. A milk fat globule membrane (MFGM) comprises polar lipids, cholesterol, proteins, glycoproteins, and enzymes [22]. The composition and structure of milk fat globules (MFG) and MFGM and their composition contribute to lipases’ access to lipids, which can result in structural changes in the MFGM [19]. Therefore, it is essential to review the effect of PEF processing on the modification of the fatty acid compositions in milk. The temperature has a significant effect on determining the rate of lipid oxidation since fats and FAs are susceptible to oxidation [55]. Lipid oxidation products are thought to be a risk factor for human health because of their mutagenic, carcinogenic, and cytotoxic characteristics [56]. Temperature influences chemical reactivity, colloidal structure organization, and stability, as well as the rate of synthesis and decomposition of hydroperoxides [57]. Despite the fact that PEF is a non-thermal technique, ohmic heating can cause temperatures to increase [12]. Ohmic or joule heating in the PEF process emerges when an electric current flows through a sample and ions and other charged molecules, such as lipids and proteins, take on the opposite charge of the electrode [56]. Earlier, McAuley et al. [22] demonstrated that PEF treatment (30 kV/cm; 22 μs; 2.4 L/min; 53 and 63 °C) had no significant effect on fat, protein, lactose, total solids, and the total solids non-fat of raw whole milk (4% fat). However, the authors observed a minor increase in the milk lipid-derived short-chain FFAs, namely butanoic (C4:0), hexanoic (C6:0), and octanoic (C8:0) acids, during two weeks of refrigerated storage at 4 and 8 °C, due to the greater rate of milk triglyceride hydrolysis by milk-derived lipases and/or esterases (lipoprotein lipase, LPL) and milk microbiota [22]. LPL is the main native milk enzyme. It only becomes active on the fat in natural MFGs when physical treatments have broken them down or if certain blood serum lipoproteins are present [58]. Nevertheless, they propose that the LPL activity is typically less active in milk fat because it is being sheltered by the MFGM and glycoproteins in the skim fraction that inhibit the lipolysis process. Similarly, the changes in FA profiles were also observed by other researchers [30]. They found a significant reduction (p < 0.05) in total SFAs and total PUFAs, while the number of total MUFAs increased in PEF-treated goat milk (20, 30, and 40 kV/cm; 5 and 10 μs; 2.5 L/h; 30 °C). The authors suggest that the changes in FA may have been caused by the breakdown of fat and triacylglycerol from MFGM and the catalytic oxidation of heterogeneous metals (Fe and Ni) from the PEF electrode material, which resulted in significant decreases in various FAs. Moreover, some other studies reported that nickel showed the best isomerization properties for FA [59]. According to Ghnimi et al. [57], heterogeneous catalysis by metal oxides can initiate FA oxidation by generating micelles comprising primary hydroperoxide, water, and other amphiphilic compounds. They also suggest that the metal oxide can provide a surface site catalyst for hydrocarbon deprotonation on the nanoscale since the C–H bonds of hydrocarbons are very weakly acidic and unfit for hydrocarbon deprotonation. Despite that, further investigation is needed to understand the PEF electroporation and heterogeneous metal catalysis effects on fatty acid molecules. According to Yang et al. [19], PEF treatment at 16 kV/cm; 30 µs; 100 mL/min; 25 °C slightly reduced the fat contents and FFA of three types of milk samples: raw milk (RM), milk with small fat globules (MS), and milk with large fat globules. Nevertheless, the same authors found that the MS with the smallest MFGs and the most modified MFGM exhibited a minor increase in FFA following the same PEF treatment. The authors proposed that the changes in FFA are likely due to the electroporation of MFGMs that exposed triglycerides to lipases and subsequently led to the release of FFA. These findings concur well with those of Garcia-Amezquita et al. [60], who found that PEF processing (36 and 42 kV/cm; 2.6 µs; 23 L/h; 25 °C) led to the clustering of small milk-fat globules, which appeared to increase the population of larger globules. However, the actual mean diameter of MFG was unaffected by PEF. It has been reported that PEF processing may result in oxidation (loss) and/or reduction (gain) in both the electrode and the electrolyte (sample) due to electrochemical reactions that took place when the interface voltage exceeded the threshold voltage [30][61][30,61]. Therefore, the decrease in fat content can be attributed to the partial loss of some fat clumps that are attached to the PEF tube or electrode.3.2. Proteins and Amino Acids
Milk proteins consist of an unstable micellar phase, known as casein, and a soluble phase of whey proteins [54]. The casein phase contains approximately 80% of the proteins and is classified as αs1, αs2, β, and κ-caseins [62]. Whey-phase proteins with the highest concentrations are α-lactalbumin (α-La) and β-lactoglobulin (β-Lg) [63]. Milk contains approximately 3.5 g/100 mL of high-quality protein, comprising nine essential amino acids required by humans [64]. Furthermore, protein components are present in the MFGM as lipoproteins, with casein having the highest content (~80%) of proteinaceous substances in bovine milk, followed by whey protein (~20%); this ratio varies by species [64]. Generally, PEF treatment affects the structure of proteins, especially the secondary and tertiary structures [65] leading to denaturation and consequent changes in their functional properties such as solubility, gelation, and aggregation. PEF has been described to affect the structure of proteins and amino acids, which are primarily associated with weaker bonds such as disulfide bonds, hydrogen bonds, and hydrophobic bonds [66]. In addition, disulfide bonds are the main covalent bonds resulting from the PEF-induced protein aggregates [50]. As a result, PEF may promote protein augmentation via hydrophobic and thiol or disulfide reaction aggregation, altering protein thermal stability and enzymatic digestibility [67]. Additionally, it has been demonstrated that some chemical bonds, such as peptides and non-covalent bonds, as well as electrostatic, hydrophobic, and Van der Waals interactions, counterbalance the protein structure [68]. The author also suggests that there is a considerable chance that the application of an electric field may affect the conformation of proteins, due to the presence of electrostatic forces in the peptide chain. These have a good correlation with Yu et al. [53], who found that the alteration of the enzyme by PEF may have reduced the proteolytic enzyme characteristics of milk, resulting in less peptide and amino acid production in PEF-treated milk (30 kV/cm; 80–120 pulses; 6 mL/min; 50 °C). The author also discovered that an increase in pulses may cause an enzyme modification to occur more severely, and that a decrease in enzyme activity may cause a decrease in the production of peptides and free amino acids. Furthermore, Yang et al. [19] observed that the β-Lg band in milk with small fat globules (MS) was reduced following PEF treatments by 8% (9 kV/cm; 30 µs; 100 mL/min; 25 °C) and 15% (16 kV/cm; 30 µs; 100 mL/min; 25 °C), whereas raw milk (RM) and milk with large fat globules (ML) demonstrated no significant changes in the β-Lg band. Likewise, the band for α-La in MS was similarly reduced following PEF treatments by 11% and 26% at 9 and 16 kV/cm, respectively (30 µs; 100 mL/min; 25 °C); but α-La in RM showed no changes and ML showed just a minor decrease (2–5%). The authors suggest that following PEF treatments, β-Lg and α-La were primarily adsorbed to the small MFGs in MS. They also found that the primary MFGM proteins, xanthine oxidase/dehydrogenase (XO/XDH), were present in the RM sample. The ML sample has a comparable profile for the MFGM proteins; however, the MS sample exhibits a very different profile since it almost completely lacked the XO/XDH band and had additional lower molecular weight protein bands as well as numerous fuzzy protein aggregation bands [19]. The authors also suggest that the MFGM composition of small MFGs differs significantly from that of large MFGs in ML, proving that the membrane of the small MFGs underwent more profound modifications throughout the separation process. Other studies have suggested that PEF treatment can alter protein polarity and the microenvironment of amino acid residues due to the liberation of native molecular structures, including the whey protein structure [51]. The changes in the milk protein were also observed by Mathys et al. [24], who found that PEF treatment (40 kV/cm; <15 s; 30 L/h; 20–45 °C) reduced 70% of the native form of milk proteins, including immunoglobulin G (IgG), immunoglobulin A (IgA), and lactoferrin. Moreover, the authors affirm that the variations in milk proteins correspond to the specific energy of PEF treatment. This suggests that high intensity of PEF treatment (>30 kV/cm) along with a mild temperature (40–60 °C) can induce denaturation of milk proteins, possibly due to the unfolding of protein molecules or their positioning towards an applied electric field. These correlate favourably with Sui et al. [52], who perceived significant changes in the concentration of natively folded lactoferrin, aggregated protein, and surface hydrophobicity of whey protein isolate after PEF treatment (30–35 kV/cm, 19.2 and 211 µs; 760 mL/min; 60–70 °C). Accordingly, Sharma et al. [69] also reported that the high intensity of PEF could increase the PEF processing temperatures due to higher specific energy, which may lead to the unfolding of protein molecules, the alignment of proteins towards electric fields, and transitions in the structure of the native proteins. They proposed that PEF-induced protein conformational changes are due to denaturation and the formation of intermolecular structures such as β-Lg or κ-casein following cross-linking between casein and serum proteins. In another study, the same authors [21] observed a decrease in MFG size, an increase in ζ-potential, specific surface area, and plasma protein absorption on the MFGM surface of PEF-treated bovine whole milk (20 and 26 kV/cm; 34 μs; 4.2 mL/s; 17–19.4 °C). It has been asserted that the superficial coverage of MFGM through plasma proteins was associated with the increase in ζ-potential, where the ζ-potential is an indication of the degree of damage to the surface of MFGM [70]. Moreover, the decrease in the size of MFG may be attributable to the shear force effect during pumping. These are in agreement with Hemar et al. [28], who reported the shear effect produced during circulating milk through the PEF system that can lead to a partial separation of casein micelles. However, the authors claimed that the effects of higher temperatures (63 °C for 30 min; 73 °C for 15 s) outweighed the modifications brought about by PEF treatment. In contrast, Liu et al. [23] found that PEF treatment (49 kV/cm; 19.36 s; 240 mL/min; 20 °C) did not affect the physicochemical properties of milk caseins, the size and ζ-potential of milk particles, the status of the whey protein, or the amount of protein in the serum. The variable medium (sample), processing settings, and PEF system used in the study can be blamed for this rather paradoxical outcome. Nevertheless, these findings appear to be well substantiated by Schottroff et al. [48], who obtained stable immunoglobulin M (IgM) and immunoglobulin G (IgG) in the 10% solution of liquid whey protein treated with PEF (50 kV/cm; 3–8 µs; 5 L/h; 20–40 °C). Since the majority of whey proteins are more susceptible to heat denaturation at neutral pH than under acidic circumstances, they contend that PEF is unlikely to have an impact on soluble proteins. Additionally, the results are consistent with Sharma et al. [20], who discovered that the PEF treatment (20 and 26 kV/cm; 34 s; 4.2 mL/s; 55 °C) had no appreciable impacts on the endothermic peak of bovine whole milk at 32.2 °C. According to this, PEF could maintain protein functioning and integrity by lowering milk protein denaturation, which would lead to less absorption onto the surface of MFGM.3.3. Vitamins
Vitamins are a group of organic complexes that are needed in small quantities; however, a lack of vitamins will lead to such syndromes as scurvy and beriberi. Vitamins fall into two categories: those that are fat-soluble (such as vitamins A, D, E, and K) and others that are water-soluble (such as vitamins B and C). It has been proposed that many variables, including light, temperature, pH, oxygen availability, metal catalysts, and the inclusion of additional antioxidants or reducing agents, can affect the stability of vitamins [68]. According to earlier research, PEF treatment had a negligible impact on milk’s vitamin content [12][33][34][48][71][12,33,34,48,71]. Mohamad et al. [12] discovered no significant changes (p > 0.05) in vitamin A (as β-carotene) and B-group vitamins (thiamine, riboflavin, and niacin) in PEF-treated goat milk (20–40 kV/cm; 5–13 s; 2.5 L/min; 30 °C). Since vitamin A and B-group vitamins are known to be sensitive to a higher temperature, the higher retention value (>94%) observed in the study is likely attributable to the lower PEF processing temperature (30 °C) used in the study. The authors conclude that extended PEF treatment time and high electric field intensities lead to decreased vitamin retention values. Riener et al. [71] reported that PEF treatment (15–35 kV/cm; 12.5–75 s; 30 °C) had no significant effects (p > 0.05) on the levels of thiamin, riboflavin, retinol, and α-tocopherol in fresh bovine raw milk. The effect of PEF (18.3–27.1 kV/cm; 400 μs; 20–25 °C) on both water-soluble (thiamin, riboflavin, and ascorbic acid) and fat-soluble (cholecalciferol and tocopherol) vitamins in milk has been evaluated by Bendicho et al. [72]. The authors observed no significant changes (p > 0.05) in both classes of vitamins except for ascorbic acid. Likewise, the reduction of ascorbic acid was found to be dependent on the electric field strength, treatment time, and temperature. However, they argued that PEF provides better retention of ascorbic acid (vitamin C) compared to thermal treatment (63 and 75 °C; 15 s and 30 min). PEF treatment has also been demonstrated to effectively maintain the bioaccessibility of provitamin A (carotenoids) from fruit juices and milk beverages. For instance, Rodríguez-Roque et al. [32] observed that PEF treatment (35 kV/cm; 1800 s; 60 mL/min; 35 °C) increased the bioaccessibility of total carotenoids in fruit juices (orange, pineapple, kiwi, and mango) and milk beverages by 15%. They found that PEF treatment gives greater stability to the total carotenoids due to the inactivation of oxidative enzymes, the interruption of cell membranes and proteins, and the release of some individual carotenoids. These are consistent with Zulueta et al. [33], who obtained minimal losses of ascorbic acid and total carotenoids with only 2 and 5% destruction rates, respectively, in PEF-treated orange juice-milk beverages (25 kV/cm; 280 µs; 60 mL/min; 57 °C). Salvia-Trujillo et al. [34] also observed higher retention (95–99%) of group B vitamins (niacin, thiamin, and riboflavin) in PEF-treated fruit juice–whole milk (FJ–WM) and fruit juice–skim milk (FJ–SM) beverages (35 kV/cm; 1800 µs; 760 mL/min; <40 °C). Following this, they discovered increased vitamin C retention (>97%) in PEF-treated FJ–WM and FJ–SM beverages, as opposed to 50% retention following 21 days of storage at 4 °C. The results demonstrate that water-soluble vitamins, particularly niacin, thiamin, and riboflavin, were not oxidatively degraded by PEF. They demonstrate that water-soluble vitamins are more responsive to heat treatment than to PEF treatment by contrasting heat (90 °C) and PEF treatment (40 °C). These findings are in good agreement with those of Schottroff et al. [48], who reported that after PEF treatment (50 kV/cm; 3–8 s; 5 L/h; 20–40 °C), there were no significant losses (p > 0.05) of vitamins A and C in liquid whey protein. The findings would seem to indicate that PEF treatment does not subject vitamins to any chemical changes.3.4. Minerals
Milk contains 20 minerals that are necessary for human nutrition. The two groups of these minerals are called macro- and microminerals. These macro- and microminerals occur in milk in an equilibrium between the soluble and colloidal phases, each serving a different physiological purpose [73]. For instance, Na and K are crucial in preserving the osmotic equilibrium between blood and milk, while Mg, Ca, P, and Zn are attached to casein micelles in milk, making their content directly tied to casein content [74]. It has been reported that Mg varies by species and is frequently connected to milk protein [75]. Although milk only contains 8–9 g/L of minerals, it is nevertheless an important source of nutrition [76]. The impact of PEF on the mineral profiles of milk has not yet been extensively researched. A literature search revealed that there is still some pertinent research on the effects of PEF on milk minerals in other species of animals. For instance, Mohamad et al. [31] found that the Fe levels in goat milk treated with PEF increased significantly (p < 0.05) at 20–40 kV/cm, 5–10 s, 2.5 L/h, and 30 °C. However, after PEF treatment, it was determined that the other minerals (Cr, Ni, and Mn) were not significant (p > 0.05). The authors concluded that the electrochemical reaction that takes place during PEF treatment may have caused the metal components from the PEF electrode to dissolve into the sample. The results obtained are in close agreement with those of Gad and Jayaram [25], who investigated the release of metal particles from stainless-steel electrodes in samples of 2% partially skimmed milk. The Fe concentration rose by 500 µg/L following PEF treatment (30–40 kV/cm; 500 mL/min; 20–25 °C) as opposed to 300 µg/L in untreated samples. The authors agreed that the commercialization of PEF processing may be impacted by electrochemical reactions that happen during milk processing about safety and quality.
