Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisa Arendt | -- | 1844 | 2023-06-08 13:48:34 | | | |
| 2 | Fanny Huang | Meta information modification | 1844 | 2023-06-12 02:53:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kuziel, G.; Moore, B.N.; Arendt, L.M. Obesity and Breast Adipose Tissue Fibrosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/45344 (accessed on 07 February 2026).
Kuziel G, Moore BN, Arendt LM. Obesity and Breast Adipose Tissue Fibrosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/45344. Accessed February 07, 2026.
Kuziel, Genevra, Brittney N. Moore, Lisa M. Arendt. "Obesity and Breast Adipose Tissue Fibrosis" Encyclopedia, https://encyclopedia.pub/entry/45344 (accessed February 07, 2026).
Kuziel, G., Moore, B.N., & Arendt, L.M. (2023, June 08). Obesity and Breast Adipose Tissue Fibrosis. In Encyclopedia. https://encyclopedia.pub/entry/45344
Kuziel, Genevra, et al. "Obesity and Breast Adipose Tissue Fibrosis." Encyclopedia. Web. 08 June, 2023.
Copy Citation
Obesity is a rising health concern and is linked to a worsened breast cancer prognosis. Tumor desmoplasia, which is characterized by elevated numbers of cancer-associated fibroblasts and the deposition of fibrillar collagens within the stroma, may contribute to the aggressive clinical behavior of breast cancer in obesity. A major component of the breast is adipose tissue, and fibrotic changes in adipose tissue due to obesity may contribute to breast cancer development and the biology of the resulting tumors. Adipose tissue fibrosis is a consequence of obesity that has multiple sources.
breast cancer
obesity
mammary gland
1. Introduction
Obesity has rapidly become a global health epidemic. Current estimates show that worldwide obesity among adults has nearly tripled since 1975 [1][2]. Obesity enhances the risk of multiple types of cancer [3][4][5]. Postmenopausal women with obesity are at a higher risk for a breast cancer diagnosis, particularly hormone receptor-positive breast cancers, which express estrogen receptor alpha (ERα) and progesterone receptor (PR) [6][7]. While still rare, the risk of ERα+ breast cancer is also increased with obesity in men [8]. Hormone receptor-positive tumors can further be divided into molecular subtypes Luminal A and Luminal B based on gene expression levels of ERα, and PR, proliferation markers, and human epidermal growth factor receptor 2 (HER2) expression [9]. Luminal B subtype tumors generally have lower expression of ERα and PR than Luminal A subtype tumors, greater proliferation, are of a higher grade, and may be less responsive to endocrine therapies [10][11]. Women with obesity are more likely to develop tumors of the Luminal B subtype [12][13]. While obesity increases breast cancer risk in postmenopausal women, obesity reduces breast cancer risk in the total population of young women [14]. However, obesity enhances other risk factors in premenopausal women, such as a family history of breast cancer or inherited breast cancer 1 or 2 (BRCA1/2) mutations [15][16][17]. Although triple-negative breast cancers, which lack expression of ERα, PR, and HER2, are more frequently diagnosed in premenopausal patients [18], the impact of obesity on the risk of development of triple-negative breast cancer in premenopausal women is less clearly defined [19][20][21], which may be in part due to underlying familial risk factors that impact younger women.
Regardless of menopausal status, recent meta-analyses involving large numbers of patients have provided evidence that obesity is associated with an increased risk of recurrence of approximately 35–40% in breast cancer patients [22]. When compared with lean women, women with obesity are more likely to be diagnosed with larger, high-grade tumors [23][24][25]. Obesity is also an independent prognostic factor for developing distant metastases due to breast cancer [26], leading to shorter disease-free periods and overall survival rates [22][24][26][27]. Obesity at the time of diagnosis is also associated with decreased response to multiple cancer therapies [28][29].
Obesity has been correlated with desmoplasia in human breast tumors [30]. Desmoplastic tumors, characterized by increased numbers of cancer associated fibroblasts (CAF) and deposition of fibrillar collagens within the stroma, are associated with diminished survival in breast cancer patients [31][32][33][34]. In addition to collagen, the accumulation of other extracellular matrix (ECM) components, such as hyaluronic acid, is associated with the generation of a more robust stroma, leading to tumor growth and a worsened prognosis [35]. Preclinical models have shown that tumor accumulation of these matrix components is associated with increased tumor interstitial pressure, the collapse of tumor vasculature, and the consequent development of a hypoxic phenotype [36]. Tumor desmoplasia is associated with reduced relapse-free survival following chemotherapy [32][33].
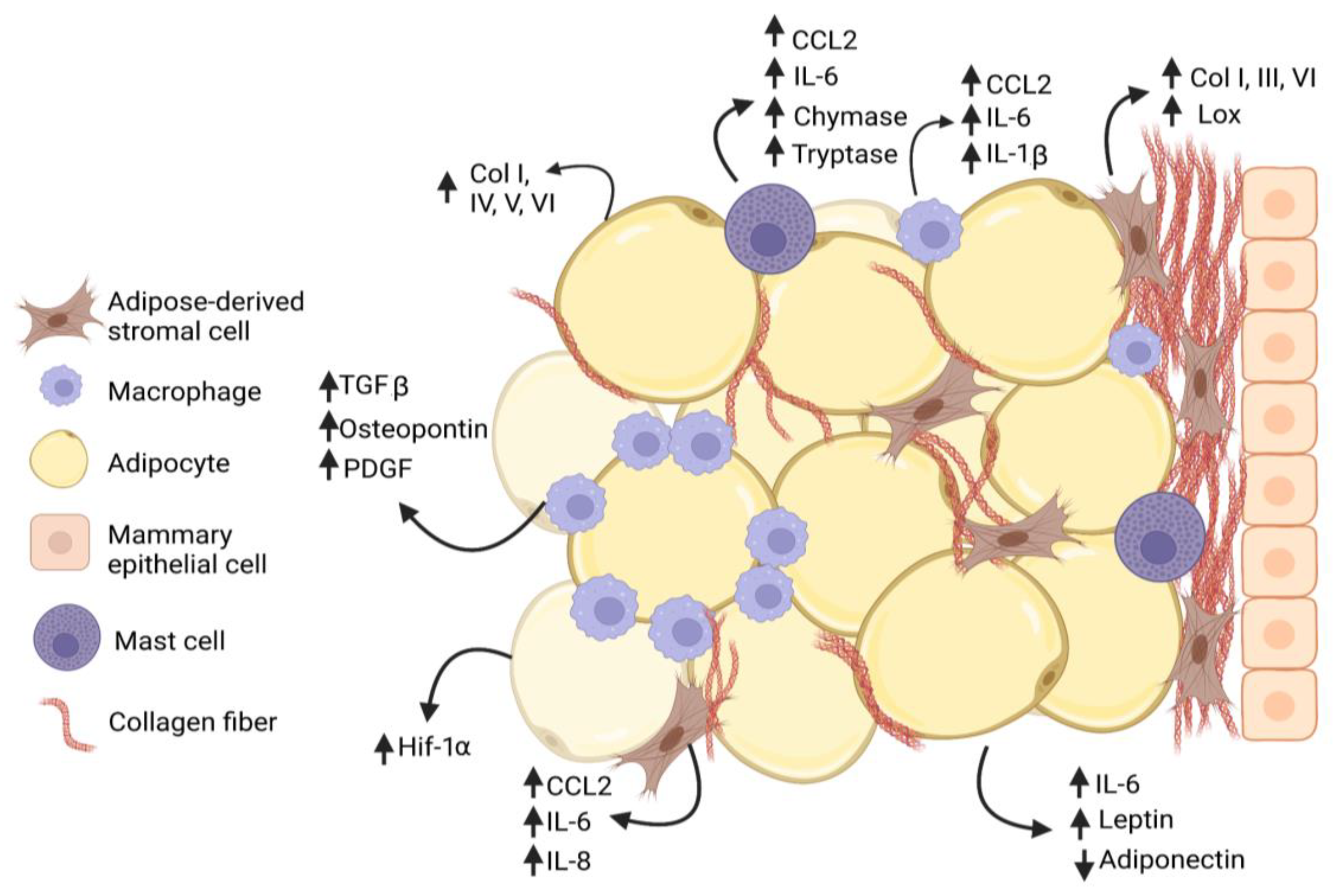
Figure 1. Interactions among cells within mammary adipose tissue enhance fibrosis in obesity. Adipose-derived stromal cells (ASC) and adipocytes increase (↑) secretion of collagen family members as well as growth factors and cytokines that promote adipose tissue fibrosis in obesity. Macrophages and mast cells are recruited into the obese adipose tissue microenvironment and interact with ASC to promote deposition of collagen and other extracellular matrix (ECM) proteins. Macrophages form characteristic crown-like structures surrounding adipocytes. Increased collagen, growth factors, and cytokines are produced by multiple cell types, while adiponectin secretion is decreased (↓) by adipocytes.
2. Obesity and Adipose Tissue Fibrosis
The percentage volume of subcutaneous adipose tissue in the breast is variable and can comprise 56% of breast weight [36]. ECM proteins in adipose tissue regulate its mechanical properties, adipogenesis, and lipid droplet growth [37][38]. During obesity, white adipose tissue undergoes expansion through adipocyte hyperplasia and hypertrophy, which are accompanied by continual remodeling of the collagen and ECM. While proper deposition of ECM supports healthy adipose tissue, increased and rigid ECM promotes the local and systemic pathologies associated with obesity [39]. Collagens accumulate around adipocytes, leading to pericellular fibrosis. In gene expression studies of subcutaneous adipose tissue from patients with obesity, fibrosis pathway genes were upregulated [40], and pericellular fibrosis was significantly enhanced [41]. In breast adipose tissue, interstitial collagen around adipocytes was significantly increased in obese patients compared to lean and overweight patients and positively correlated with body mass index (BMI) [42]. Following gastric bypass surgery for weight loss, fibrosis was negatively correlated with fat mass loss [41], suggesting that fat deposition impacts the degree of fibrosis present in adipose tissue.
Although obesity enhances fibrosis around adipocytes, within mammary tissue, obesity also increases collagen deposition around mammary ducts and preneoplastic lesions [43][44][45]. Interstitial collagen in mammary adipose tissue demonstrated elevated linearity and stiffness in both diet-induced obese mouse models and ob/ob mice, which have a mutation in the gene encoding leptin [30]. Further, breast tissue from women with obesity had increased collagen fiber alignment [30]. Expression of various collagen family members, including Col1a1, Col3a1, and Col6a1, was upregulated in the subcutaneous adipose tissue of obese mice [46]. In humans with obesity, COL5 and COL6 expression levels were elevated, and elastin levels were reduced in subcutaneous fat [47][48]. In addition to collagen family members, tenascin C and osteopontin are matricellular proteins that are upregulated in obese adipose tissue. Tenascin C expression was enhanced in subcutaneous adipose tissue of obese mice [49], and fibrosis was reduced in mice that the lack expression of tenascin C [50]. Osteopontin was upregulated 40 to 80-fold in subcutaneous adipose tissue of obese mice and was highly expressed by adipose tissue macrophages [51]. Fibronectin was also significantly increased in mammary adipose tissue of obese mice, as well as in breast tumors of obese patients [30]. Thus, obesity leads to increased collagen deposition and changes the characteristics of the ECM in the breast microenvironment.
Enhanced adipose tissue fibrosis in the context of obesity is also associated with a significant increase in tissue stiffness [52]. Cu-dependent lysyl oxidase (LOX), one of five members of the LOX family, contributes to the functions of the ECM by promoting the formation of intra- and intermolecular cross-linkages between ECM components. Elevated levels of LOX were associated with enhanced ECM stiffness [53]. LOX expression was increased in obese subcutaneous adipose tissue of mice and rats [46][54][55][56], and LOX expression in subcutaneous adipose tissue of human subjects positively correlated with BMI [56]. In the mammary fat pad of mice, injection of fibroblasts overexpressing LOX increased tissue stiffness, collagen deposition, and the linearity of collagen fibers [57]. In contrast, when LOX was inhibited, collagen deposition was decreased, and collagen fibers were less linear [57]. Interestingly, LOX expression did not significantly decrease in the adipose tissue of humans nine months after bariatric surgery [56], which may suggest that pathological alterations in fibrosis due to obesity may not be easily reversed following weight reduction.
In addition to its structural role, the ECM also serves as a reservoir for soluble factors, including chemokines. Chemokine binding to resident matrix glycosaminoglycans is important for controlling local concentrations of these soluble factors and their resistance to proteolytic activity and signaling capabilities [58][59]. An increasing number of growth factors, including transforming growth factor beta (TGFβ), insulin-like growth factor, fibroblast growth factor, and hepatocyte growth factor, have been found to associate with ECM proteins or with heparan sulfate [60]. Changes in the composition of the ECM alter the ability of the tissue to bind and retain secreted molecules. For example, TGFβ1 expression is elevated in the adipose tissue of obese mice and humans [61][62][63]. TGFβ1 is produced in a latent form that must undergo extracellular activation prior to receptor binding [64]. Within the ECM, latent TGFβ1 complexes both with latent TGFβ1 binding proteins and matrix components, including decorin, which sequester inactive TGFβ1 until activated [65]. In the obese mammary gland, decorin is enhanced within the ECM and complexes with latent TGFβ1, resulting in increased TGFβ1 storage within the ECM [66]. As TGFβ1 has been implicated in promoting epithelial-to-mesenchymal transition (EMT) in tumor cells and the growth of CAF within the tumor microenvironment [67][68], elevated TGFβ stores within the ECM may impact both the proliferating tumor cells and developing CAF populations.
Fibrosis observed in obese adipose tissue is thought to be in part a result of hypoxia [39], as expanding adipocytes no longer have sufficient blood supply to oxygenate the tissue. In both diet-induced and genetic mouse models of obesity, obese mice had significantly increased hypoxia-induced factor-1 alpha (HIF-1α) expression in adipose tissue [54][69][70]. Concurrent with increased DNA binding of HIF-1α, obese mice demonstrated increased collagen deposition around adipocytes, while treatment of obese mice with β-aminopropionitrile, a LOX family inhibitor, significantly reduced adipose tissue fibrosis [54]. Similarly, collagen deposition around adipocytes and LOX expression was decreased in adipose tissue when HIF-1α was inhibited in obese mice using targeted inhibitors or overexpression of a dominant negative HIF-1α mutant in adipose tissue [70]. As elevated levels of HIF-1α in breast tumors have been associated with metabolic changes in tumor cells, metastasis, and chemotherapy resistance [71], HIF-1α upregulation within the mammary microenvironment prior to tumor formation may contribute to the growth of more aggressive tumors (Figure 1). As in obese adipose tissue, tumor vasculature is significantly more permeable than that of normal tissue [72], which is a characteristic that promotes both immune cell extravasation and fibrosis.
Excess collagen within the mammary gland may enhance cancer risk through the promotion of an aggressive phenotype in premalignant cells. In vitro, MCF10A cells, which model normal breast epithelium, and premalignant MCF10AT cells were more proliferative and invasive when grown on stiff ECM [30][73]. In vivo, injection of MCF10AT cells into mammary fat pads conditioned with LOX-overexpressing fibroblasts led to increased tumor growth and invasion [57]. Using the expression of MMTV-PyMT in a transgenic mouse model of breast density, increased density of mammary collagen enhanced the growth of invasive, rapidly metastatic tumors [73]. Once preneoplastic lesions form, increased collagen deposition and tissue stiffness may also enhance cancer stem-like cells [74][75], which have been implicated in treatment resistance, disease recurrence, and metastasis [76]. Consistent with this, the transplantation of ERα+ mammary tumor cells into collagen-dense mammary glands led to increased cancer stem-like cells, circulating tumor cells, and metastasis [77][78]. Elevated collagen deposition and tissue stiffness can also cause aberrant function of endothelial cells, leading to disrupted barrier function and elevated permeability [79]. These defects in endothelial cell function can enhance the release of tumor cells into the circulation and promote metastasis. Together, these results suggest that increased collagen deposition in breast tissue may be implicated in the early promotion of the growth of obesity-associated tumors (Figure 2).
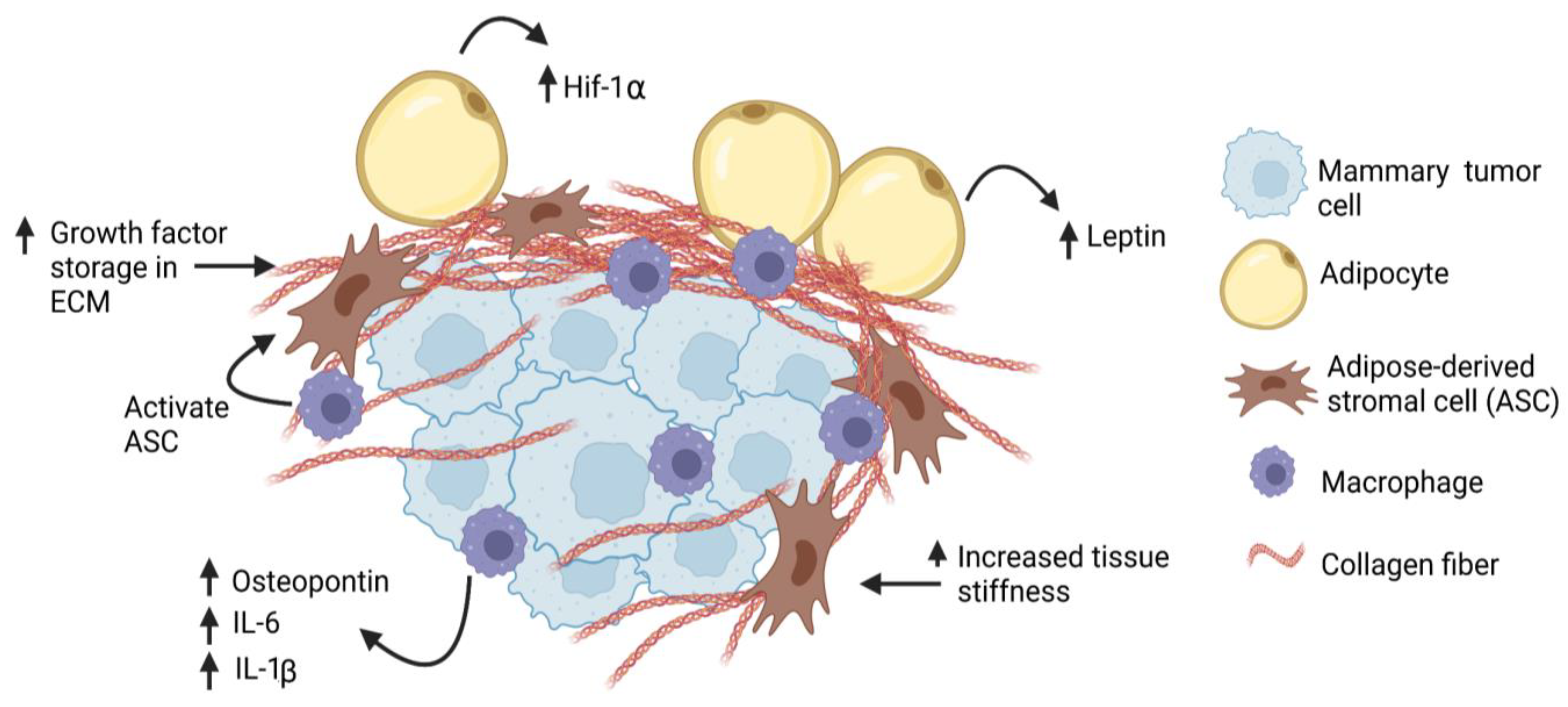
Figure 2. Obesity increases desmoplasia in mammary tumor microenvironment. Tumor-associated macrophages may activate adipose-derived stromal cells (ASC), which may then contribute to the CAF population. Through production of thicker and stiffer extracellular matrix (ECM), breast cancer cells may have increased (↑) exposure to growth factors, hypoxia (Hif-1α), and cytokines, leading to increased proliferation and invasion.
References
- World Health Organization, Breast Cancer Fact Sheet. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 31 January 2023).
- National Institute of Diabetes and Digestive and Kidney Diseases, Overweight and Obesity Statistics. 2021. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity (accessed on 31 January 2023).
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565.
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638.
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and cancer: A current overview of epidemiology, pathogenesis, outcomes, and management. Cancers 2023, 15, 485.
- Suzuki, R.; Orsini, N.; Saji, S.; Key, T.J.; Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int. J. Cancer 2009, 124, 698–712.
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136.
- Brinton, L.A.; Cook, M.B.; McCormack, V.; Johnson, K.C.; Olsson, H.; Casagrande, J.T.; Cooke, R.; Falk, R.T.; Gapstur, S.M.; Gaudet, M.M.; et al. Anthropometric and hormonal risk factors for male breast cancer: Male breast cancer pooling project results. J. Natl. Cancer Inst. 2014, 106, djt465.
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between luminal A and luminal B subtypes according to Ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clin. Med. Insights. Oncol. 2014, 8, 107–111.
- Ahn, H.J.; Jung, S.J.; Kim, T.H.; Oh, M.K.; Yoon, H.K. Differences in clinical outcomes between luminal A and B type breast cancers according to the St. Gallen Consensus 2013. J. Breast Cancer 2015, 18, 149–159.
- Hashmi, A.A.; Aijaz, S.; Khan, S.M.; Mahboob, R.; Irfan, M.; Zafar, N.I.; Nisar, M.; Siddiqui, M.; Edhi, M.M.; Faridi, N.; et al. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J. Surg. Oncol. 2018, 16, 1.
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167.
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Douglas, T.W.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597.
- Premenopausal Breast Cancer Collaborative Group. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncol. 2018, 4, e181771.
- Carpenter, C.L.; Ross, R.K.; Paganini-Hill, A.; Bernstein, L. Effect of family history, obesity and exercise on breast cancer risk among postmenopausal women. Int. J. Cancer 2003, 106, 96–102.
- Kotsopoulos, J.; Olopado, O.I.; Ghadirian, P.; Lubinski, J.; Lynch, H.T.; Isaacs, C.; Weber, B.; Kim-Sing, C.; Ainsworth, P.; Foulkes, W.D.; et al. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2005, 7, R833–R843.
- Nkondjock, A.; Robidoux, A.; Paredes, Y.; Narod, S.A.; Ghadirian, P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res. Treat. 2006, 98, 285–294.
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist 2014, 19, 1076–1083.
- Dolle, J.M.; Daling, J.R.; White, E.; Brinton, L.A.; Doody, D.R.; Porter, P.L.; Malone, K.E. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1157–1166.
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314.
- Yang, X.R.; Sherman, M.E.; Rimm, D.L.; Lissowska, J.; Brinton, L.A.; Peplonska, B.; Hewitt, S.M.; Anderson, W.F.; Szeszenia-Dabrowska, N.; Bardin-Mikolajczak, A.; et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 439–443.
- Jiralerspong, S.; Goodwin, P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016, 34, 4203–4216.
- Carmichael, A.R. Obesity as a risk factor for development and poor prognosis of breast cancer. BJOG 2006, 113, 1160–1166.
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591.
- Mazzarella, L.; Disalvatore, D.; Bagnardi, V.; Rotmensz, N.; Galbiati, D.; Caputo, S.; Curigliano, G.; Pelicci, P.G. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur. J. Cancer 2013, 49, 3588–3597.
- Ewertz, M.; Jensen, M.B.; Gunnarsdottir, K.A.; Hojris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31.
- Majed, B.; Moreau, T.; Senouci, K.; Salmon, R.J.; Fourquet, A.; Asselain, B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res. Treat. 2008, 111, 329–342.
- Karatas, F.; Erdem, G.U.; Sahin, S.; Aytekin, A.; Yuce, D.; Sever, A.R.; Babacan, T.; Ates, O.; Ozisik, Y.; Altundag, K. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 2017, 32, 237–244.
- Ioannides, S.J.; Barlow, P.L.; Elwood, J.M.; Porter, D. Effect of obesity on aromatase inhibitor efficacy in postmenopausal, hormone receptor-positive breast cancer: A systematic review. Breast Cancer Res. Treat. 2014, 147, 237–248.
- Seo, B.R.; Bhardwaj, P.; Choi, S.; Gonzalez, J.; Andresen Eguiluz, R.C.; Wang, K.; Mohanan, S.; Morris, P.G.; Du, B.; Zhou, X.K.; et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 2015, 7, 301ra130.
- Beck, A.H.; Sangoi, A.R.; Leung, S.; Marinelli, R.J.; Nielsen, T.O.; van de Vijver, M.J.; West, R.B.; van de Rijn, M.; Koller, D. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci. Transl. Med. 2011, 3, 108ra113.
- de Kruijf, E.M.; van Nes, J.G.; van de Velde, C.J.; Putter, H.; Smit, V.T.; Liefers, G.J.; Kuppen, P.J.; Tollenaar, R.A.; Mesker, W.E. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res. Treat. 2011, 125, 687–696.
- Dekker, T.J.; van de Velde, C.J.; van Pelt, G.W.; Kroep, J.R.; Julien, J.P.; Smit, V.T.; Tollenaar, R.A.; Mesker, W.E. Prognostic significance of the tumor-stroma ratio: Validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res. Treat. 2013, 139, 371–379.
- Moorman, A.M.; Vink, R.; Heijmans, H.J.; van der Palen, J.; Kouwenhoven, E.A. The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur. J. Surg. Oncol. 2012, 38, 307–313.
- Whatcott, C.J.; Diep, C.H.; Jiang, P.; Watanabe, A.; LoBello, J.; Sima, C.; Hostetter, G.; Shepard, H.M.; Von Hoff, D.D.; Han, H. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 2015, 21, 3561–3568.
- Friman, T.; Gustafsson, R.; Stuhr, L.B.; Chidiac, J.; Heldin, N.E.; Reed, R.K.; Oldberg, A.; Rubin, K. Increased fibrosis and interstitial fluid pressure in two different types of syngeneic murine carcinoma grown in integrin β3-subunit deficient mice. PLoS ONE 2012, 7, e34082.
- Alkhouli, N.; Mansfield, J.; Green, E.; Bell, J.; Knight, B.; Liversedge, N.; Tham, J.C.; Welbourn, R.; Shore, A.C.; Kos, K.; et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1427–E1435.
- Nakajima, I.; Muroya, S.; Tanabe, R.; Chikuni, K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002, 70, 84–91.
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell. Metab. 2013, 18, 470–477.
- Vila, I.K.; Badin, P.M.; Marques, M.A.; Monbrun, L.; Lefort, C.; Mir, L.; Louche, K.; Bourlier, V.; Roussel, B.; Gui, P.; et al. Immune cell toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell. Rep. 2014, 7, 1116–1129.
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825.
- Springer, N.L.; Iyengar, N.M.; Bareja, R.; Verma, A.; Jochelson, M.S.; Giri, D.D.; Zhou, X.K.; Elemento, O.; Dannenberg, A.J.; Fischbach, C. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am. J. Pathol. 2019, 189, 2019–2035.
- Kamikawa, A.; Ichii, O.; Yamaji, D.; Imao, T.; Suzuki, C.; Okamatsu-Ogura, Y.; Terao, A.; Kon, Y.; Kimura, K. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev. Dyn. 2009, 238, 1092–1099.
- Wolfson, B.; Zhang, Y.; Gernapudi, R.; Duru, N.; Yao, Y.; Lo, P.K.; Zhou, Q. A high-fat diet promotes mammary gland myofibroblast differentiation through microRNA 140 downregulation. Mol. Cell. Biol. 2017, 37, e00461-16.
- Chamberlin, T.; Clack, M.; Silvers, C.; Kuziel, G.; Thompson, V.; Johnson, H.; Arendt, L.M. Targeting obesity-induced macrophages during preneoplastic growth promotes mammary epithelial stem/progenitor activity, DNA damage, and tumor formation. Cancer Res. 2020, 80, 4465–4475.
- Marcelin, G.; Ferreira, A.; Liu, Y.; Atlan, M.; Aron-Wisnewsky, J.; Pelloux, V.; Botbol, Y.; Ambrosini, M.; Fradet, M.; Rouault, C.; et al. A PDGFRα-mediated switch toward CD9high adipocyte progenitors controls obesity-induced adipose tissue fibrosis. Cell. Metab. 2017, 25, 673–685.
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1016–E1027.
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591.
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Frühbeck, G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889.
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703.
- Kiefer, F.W.; Zeyda, M.; Todoric, J.; Huber, J.; Geyeregger, R.; Weichhart, T.; Aszmann, O.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinol 2008, 149, 1350–1357.
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.L.; Torcivia, A.; Sasso, M.; et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: Links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907.
- Wang, T.H.; Hsia, S.M.; Shieh, T.M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016, 18, 62.
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483.
- Miana, M.; Galan, M.; Martinez-Martinez, E.; Varona, S.; Jurado-Lopez, R.; Bausa-Miranda, B.; Antequera, A.; Luaces, M.; Martinez-Gonzalez, J.; Rodriguez, C.; et al. The lysyl oxidase inhibitor β-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats. Dis. Model. Mech. 2015, 8, 543–551.
- Pastel, E.; Price, E.; Sjöholm, K.; McCulloch, L.J.; Rittig, N.; Liversedge, N.; Knight, B.; Møller, N.; Svensson, P.A.; Kos, K. Lysyl oxidase and adipose tissue dysfunction. Metabolism 2018, 78, 118–127.
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906.
- Proudfoot, A.E.I.; Johnson, Z.; Bonvin, P.; Handel, T.M. Glycosaminoglycan interactions with chemokines add complexity to a complex system. Pharmaceuticals 2017, 10, 70.
- Monneau, Y.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The sweet spot: How GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2016, 99, 935–953.
- Taipale, J.; Keski-Oja, J. Growth factors in the extracellular matrix. FASEB J. 1997, 11, 51–59.
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 1997, 3, 37–48.
- Alessi, M.C.; Bastelica, D.; Morange, P.; Berthet, B.; Leduc, I.; Verdier, M.; Geel, O.; Juhan-Vague, I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000, 49, 1374–1380.
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005, 54, 1546–1551.
- Moses, H.L.; Coffey, R.J., Jr.; Leof, E.B.; Lyons, R.M.; Keski-Oja, J. Transforming growth factor beta regulation of cell proliferation. J. Cell. Physiol. Suppl. 1987, 133 (Suppl. S5), 1–7.
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-β function. J. Biochem. 2012, 152, 321–329.
- Chamberlin, T.; Thompson, V.; Hillers-Ziemer, L.E.; Walton, B.N.; Arendt, L.M. Obesity reduces mammary epithelial cell TGFβ1 activity through macrophage-mediated extracellular matrix remodeling. FASEB J. 2020, 34, 8611–8624.
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell. Res. 2009, 19, 156–172.
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176.
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2008, 32, 451–463.
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 2013, 33, 904–917.
- Yong, L.; Tang, S.; Yu, H.; Zhang, H.; Zhang, Y.; Wan, Y.; Cai, F. The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer. Front. Oncol. 2022, 12, 964934.
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Seo, K.; Yamashita, H.; Hosoya, Y.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. In vivo imaging in mice reveals local cell dynamics in inflammation in obese adipose tissue. J. Clin. Investig. 2008, 11, 710–721.
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11.
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The role of the extracellular matrix in cancer stemness. Front. Cell. Dev. Biol. 2019, 7, 86.
- Shan, N.L.; Shin, Y.; Yang, G.; Furmanski, P.; Suh, N. Breast cancer stem cells: A review of their characteristics and the agents that affect them. Mol. Carcinog. 2021, 60, 73–100.
- Song, K.; Farzaneh, M. Signaling pathways governing breast cancer cells behavior. Stem. Cell. Res. Ther. 2021, 12, 245.
- Barcus, C.E.; O’Leary, K.A.; Brockman, J.L.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017, 19, 9.
- Shea, M.P.; O’Leary, K.A.; Wegner, K.A.; Vezina, C.M.; Schuler, L.A. High collagen density augments mTOR-dependent cancer stem cells in ERα+ mammary carcinomas and increases mTOR-independent lung metastases. Cancer Lett. 2018, 433, 1–9.
- Zanotelli, M.R.; Reinhart-King, C.A. Mechanical forces in tumor angiogenesis. Adv. Exp. Med. Biol. 2018, 1092, 91–112.
More
Information
Subjects:
Pathology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
671
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




