Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pathology
Obesity is a rising health concern and is linked to a worsened breast cancer prognosis. Tumor desmoplasia, which is characterized by elevated numbers of cancer-associated fibroblasts and the deposition of fibrillar collagens within the stroma, may contribute to the aggressive clinical behavior of breast cancer in obesity. A major component of the breast is adipose tissue, and fibrotic changes in adipose tissue due to obesity may contribute to breast cancer development and the biology of the resulting tumors. Adipose tissue fibrosis is a consequence of obesity that has multiple sources.
- breast cancer
- obesity
- mammary gland
1. Introduction
Obesity has rapidly become a global health epidemic. Current estimates show that worldwide obesity among adults has nearly tripled since 1975 [1,2]. Obesity enhances the risk of multiple types of cancer [3,4,5]. Postmenopausal women with obesity are at a higher risk for a breast cancer diagnosis, particularly hormone receptor-positive breast cancers, which express estrogen receptor alpha (ERα) and progesterone receptor (PR) [6,7]. While still rare, the risk of ERα+ breast cancer is also increased with obesity in men [8]. Hormone receptor-positive tumors can further be divided into molecular subtypes Luminal A and Luminal B based on gene expression levels of ERα, and PR, proliferation markers, and human epidermal growth factor receptor 2 (HER2) expression [9]. Luminal B subtype tumors generally have lower expression of ERα and PR than Luminal A subtype tumors, greater proliferation, are of a higher grade, and may be less responsive to endocrine therapies [10,11]. Women with obesity are more likely to develop tumors of the Luminal B subtype [12,13]. While obesity increases breast cancer risk in postmenopausal women, obesity reduces breast cancer risk in the total population of young women [14]. However, obesity enhances other risk factors in premenopausal women, such as a family history of breast cancer or inherited breast cancer 1 or 2 (BRCA1/2) mutations [15,16,17]. Although triple-negative breast cancers, which lack expression of ERα, PR, and HER2, are more frequently diagnosed in premenopausal patients [18], the impact of obesity on the risk of development of triple-negative breast cancer in premenopausal women is less clearly defined [19,20,21], which may be in part due to underlying familial risk factors that impact younger women.
Regardless of menopausal status, recent meta-analyses involving large numbers of patients have provided evidence that obesity is associated with an increased risk of recurrence of approximately 35–40% in breast cancer patients [22]. When compared with lean women, women with obesity are more likely to be diagnosed with larger, high-grade tumors [23,24,25]. Obesity is also an independent prognostic factor for developing distant metastases due to breast cancer [26], leading to shorter disease-free periods and overall survival rates [22,24,26,27]. Obesity at the time of diagnosis is also associated with decreased response to multiple cancer therapies [28,29].
Obesity has been correlated with desmoplasia in human breast tumors [30]. Desmoplastic tumors, characterized by increased numbers of cancer associated fibroblasts (CAF) and deposition of fibrillar collagens within the stroma, are associated with diminished survival in breast cancer patients [31,32,33,34]. In addition to collagen, the accumulation of other extracellular matrix (ECM) components, such as hyaluronic acid, is associated with the generation of a more robust stroma, leading to tumor growth and a worsened prognosis [35]. Preclinical models have shown that tumor accumulation of these matrix components is associated with increased tumor interstitial pressure, the collapse of tumor vasculature, and the consequent development of a hypoxic phenotype [36]. Tumor desmoplasia is associated with reduced relapse-free survival following chemotherapy [32,33].
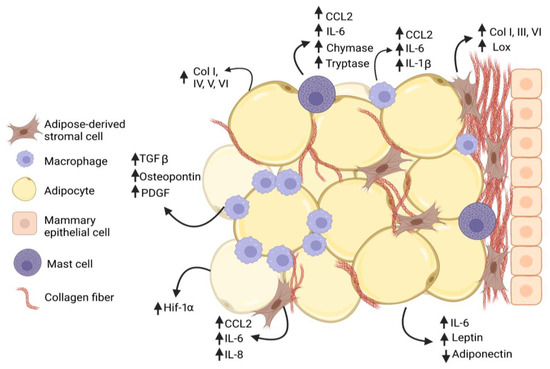
Figure 1. Interactions among cells within mammary adipose tissue enhance fibrosis in obesity. Adipose-derived stromal cells (ASC) and adipocytes increase (↑) secretion of collagen family members as well as growth factors and cytokines that promote adipose tissue fibrosis in obesity. Macrophages and mast cells are recruited into the obese adipose tissue microenvironment and interact with ASC to promote deposition of collagen and other extracellular matrix (ECM) proteins. Macrophages form characteristic crown-like structures surrounding adipocytes. Increased collagen, growth factors, and cytokines are produced by multiple cell types, while adiponectin secretion is decreased (↓) by adipocytes.
2. Obesity and Adipose Tissue Fibrosis
The percentage volume of subcutaneous adipose tissue in the breast is variable and can comprise 56% of breast weight [36]. ECM proteins in adipose tissue regulate its mechanical properties, adipogenesis, and lipid droplet growth [37,38]. During obesity, white adipose tissue undergoes expansion through adipocyte hyperplasia and hypertrophy, which are accompanied by continual remodeling of the collagen and ECM. While proper deposition of ECM supports healthy adipose tissue, increased and rigid ECM promotes the local and systemic pathologies associated with obesity [39]. Collagens accumulate around adipocytes, leading to pericellular fibrosis. In gene expression studies of subcutaneous adipose tissue from patients with obesity, fibrosis pathway genes were upregulated [40], and pericellular fibrosis was significantly enhanced [41]. In breast adipose tissue, interstitial collagen around adipocytes was significantly increased in obese patients compared to lean and overweight patients and positively correlated with body mass index (BMI) [42]. Following gastric bypass surgery for weight loss, fibrosis was negatively correlated with fat mass loss [41], suggesting that fat deposition impacts the degree of fibrosis present in adipose tissue.
Although obesity enhances fibrosis around adipocytes, within mammary tissue, obesity also increases collagen deposition around mammary ducts and preneoplastic lesions [43,44,45]. Interstitial collagen in mammary adipose tissue demonstrated elevated linearity and stiffness in both diet-induced obese mouse models and ob/ob mice, which have a mutation in the gene encoding leptin [30]. Further, breast tissue from women with obesity had increased collagen fiber alignment [30]. Expression of various collagen family members, including Col1a1, Col3a1, and Col6a1, was upregulated in the subcutaneous adipose tissue of obese mice [46]. In humans with obesity, COL5 and COL6 expression levels were elevated, and elastin levels were reduced in subcutaneous fat [47,48]. In addition to collagen family members, tenascin C and osteopontin are matricellular proteins that are upregulated in obese adipose tissue. Tenascin C expression was enhanced in subcutaneous adipose tissue of obese mice [49], and fibrosis was reduced in mice that the lack expression of tenascin C [50]. Osteopontin was upregulated 40 to 80-fold in subcutaneous adipose tissue of obese mice and was highly expressed by adipose tissue macrophages [51]. Fibronectin was also significantly increased in mammary adipose tissue of obese mice, as well as in breast tumors of obese patients [30]. Thus, obesity leads to increased collagen deposition and changes the characteristics of the ECM in the breast microenvironment.
Enhanced adipose tissue fibrosis in the context of obesity is also associated with a significant increase in tissue stiffness [52]. Cu-dependent lysyl oxidase (LOX), one of five members of the LOX family, contributes to the functions of the ECM by promoting the formation of intra- and intermolecular cross-linkages between ECM components. Elevated levels of LOX were associated with enhanced ECM stiffness [53]. LOX expression was increased in obese subcutaneous adipose tissue of mice and rats [46,54,55,56], and LOX expression in subcutaneous adipose tissue of human subjects positively correlated with BMI [56]. In the mammary fat pad of mice, injection of fibroblasts overexpressing LOX increased tissue stiffness, collagen deposition, and the linearity of collagen fibers [57]. In contrast, when LOX was inhibited, collagen deposition was decreased, and collagen fibers were less linear [57]. Interestingly, LOX expression did not significantly decrease in the adipose tissue of humans nine months after bariatric surgery [56], which may suggest that pathological alterations in fibrosis due to obesity may not be easily reversed following weight reduction.
In addition to its structural role, the ECM also serves as a reservoir for soluble factors, including chemokines. Chemokine binding to resident matrix glycosaminoglycans is important for controlling local concentrations of these soluble factors and their resistance to proteolytic activity and signaling capabilities [58,59]. An increasing number of growth factors, including transforming growth factor beta (TGFβ), insulin-like growth factor, fibroblast growth factor, and hepatocyte growth factor, have been found to associate with ECM proteins or with heparan sulfate [60]. Changes in the composition of the ECM alter the ability of the tissue to bind and retain secreted molecules. For example, TGFβ1 expression is elevated in the adipose tissue of obese mice and humans [61,62,63]. TGFβ1 is produced in a latent form that must undergo extracellular activation prior to receptor binding [64]. Within the ECM, latent TGFβ1 complexes both with latent TGFβ1 binding proteins and matrix components, including decorin, which sequester inactive TGFβ1 until activated [65]. In the obese mammary gland, decorin is enhanced within the ECM and complexes with latent TGFβ1, resulting in increased TGFβ1 storage within the ECM [66]. As TGFβ1 has been implicated in promoting epithelial-to-mesenchymal transition (EMT) in tumor cells and the growth of CAF within the tumor microenvironment [67,68], elevated TGFβ stores within the ECM may impact both the proliferating tumor cells and developing CAF populations.
Fibrosis observed in obese adipose tissue is thought to be in part a result of hypoxia [39], as expanding adipocytes no longer have sufficient blood supply to oxygenate the tissue. In both diet-induced and genetic mouse models of obesity, obese mice had significantly increased hypoxia-induced factor-1 alpha (HIF-1α) expression in adipose tissue [54,69,70]. Concurrent with increased DNA binding of HIF-1α, obese mice demonstrated increased collagen deposition around adipocytes, while treatment of obese mice with β-aminopropionitrile, a LOX family inhibitor, significantly reduced adipose tissue fibrosis [54]. Similarly, collagen deposition around adipocytes and LOX expression was decreased in adipose tissue when HIF-1α was inhibited in obese mice using targeted inhibitors or overexpression of a dominant negative HIF-1α mutant in adipose tissue [70]. As elevated levels of HIF-1α in breast tumors have been associated with metabolic changes in tumor cells, metastasis, and chemotherapy resistance [71], HIF-1α upregulation within the mammary microenvironment prior to tumor formation may contribute to the growth of more aggressive tumors (Figure 1). As in obese adipose tissue, tumor vasculature is significantly more permeable than that of normal tissue [72], which is a characteristic that promotes both immune cell extravasation and fibrosis.
Excess collagen within the mammary gland may enhance cancer risk through the promotion of an aggressive phenotype in premalignant cells. In vitro, MCF10A cells, which model normal breast epithelium, and premalignant MCF10AT cells were more proliferative and invasive when grown on stiff ECM [30,73]. In vivo, injection of MCF10AT cells into mammary fat pads conditioned with LOX-overexpressing fibroblasts led to increased tumor growth and invasion [57]. Using the expression of MMTV-PyMT in a transgenic mouse model of breast density, increased density of mammary collagen enhanced the growth of invasive, rapidly metastatic tumors [73]. Once preneoplastic lesions form, increased collagen deposition and tissue stiffness may also enhance cancer stem-like cells [74,75], which have been implicated in treatment resistance, disease recurrence, and metastasis [76]. Consistent with this, the transplantation of ERα+ mammary tumor cells into collagen-dense mammary glands led to increased cancer stem-like cells, circulating tumor cells, and metastasis [77,78]. Elevated collagen deposition and tissue stiffness can also cause aberrant function of endothelial cells, leading to disrupted barrier function and elevated permeability [79]. These defects in endothelial cell function can enhance the release of tumor cells into the circulation and promote metastasis. Together, these results suggest that increased collagen deposition in breast tissue may be implicated in the early promotion of the growth of obesity-associated tumors (Figure 2).
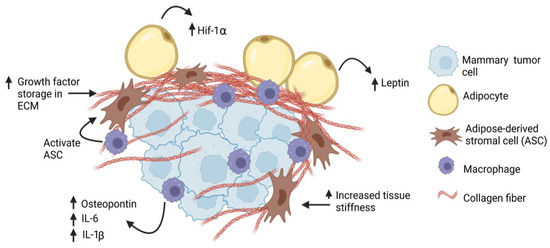
Figure 2. Obesity increases desmoplasia in mammary tumor microenvironment. Tumor-associated macrophages may activate adipose-derived stromal cells (ASC), which may then contribute to the CAF population. Through production of thicker and stiffer extracellular matrix (ECM), breast cancer cells may have increased (↑) exposure to growth factors, hypoxia (Hif-1α), and cytokines, leading to increased proliferation and invasion.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15112929
This entry is offline, you can click here to edit this entry!
