
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pradeep Tyagi | -- | 2629 | 2023-06-07 15:36:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 2629 | 2023-06-08 06:15:34 | | |
Video Upload Options
While the poor resolution of soft tissue obtained by widely available imaging options such as abdominal sonography and radiation-based CT leaves them only suitable for measuring the gross tumor volume and bladder wall thickening, dynamic contrast-enhanced magnetic resolution imaging (DCE MRI) is demonstrably superior in resolving muscle invasion. However, major barriers still exist in its adoption. Instead of injection for DCE-MRI, intravesical contrast-enhanced MRI (ICE-MRI) instills Gadolinium chelate (Gadobutrol) together with trace amounts of superparamagnetic agents for measurement of tumor volume, depth, and aggressiveness. ICE-MRI leverages leaky tight junctions to accelerate passive paracellular diffusion of Gadobutrol (604.71 Daltons) by treading the paracellular ingress pathway of fluorescein sodium and of mitomycin (<400 Daltons) into bladder tumor. The soaring cost of diagnosis and care of bladder cancer could be mitigated by reducing the use of expensive operating room resources with a potential non-surgical imaging option for cancer surveillance, thereby reducing over-diagnosis and over-treatment and increasing organ preservation.
1. Introduction
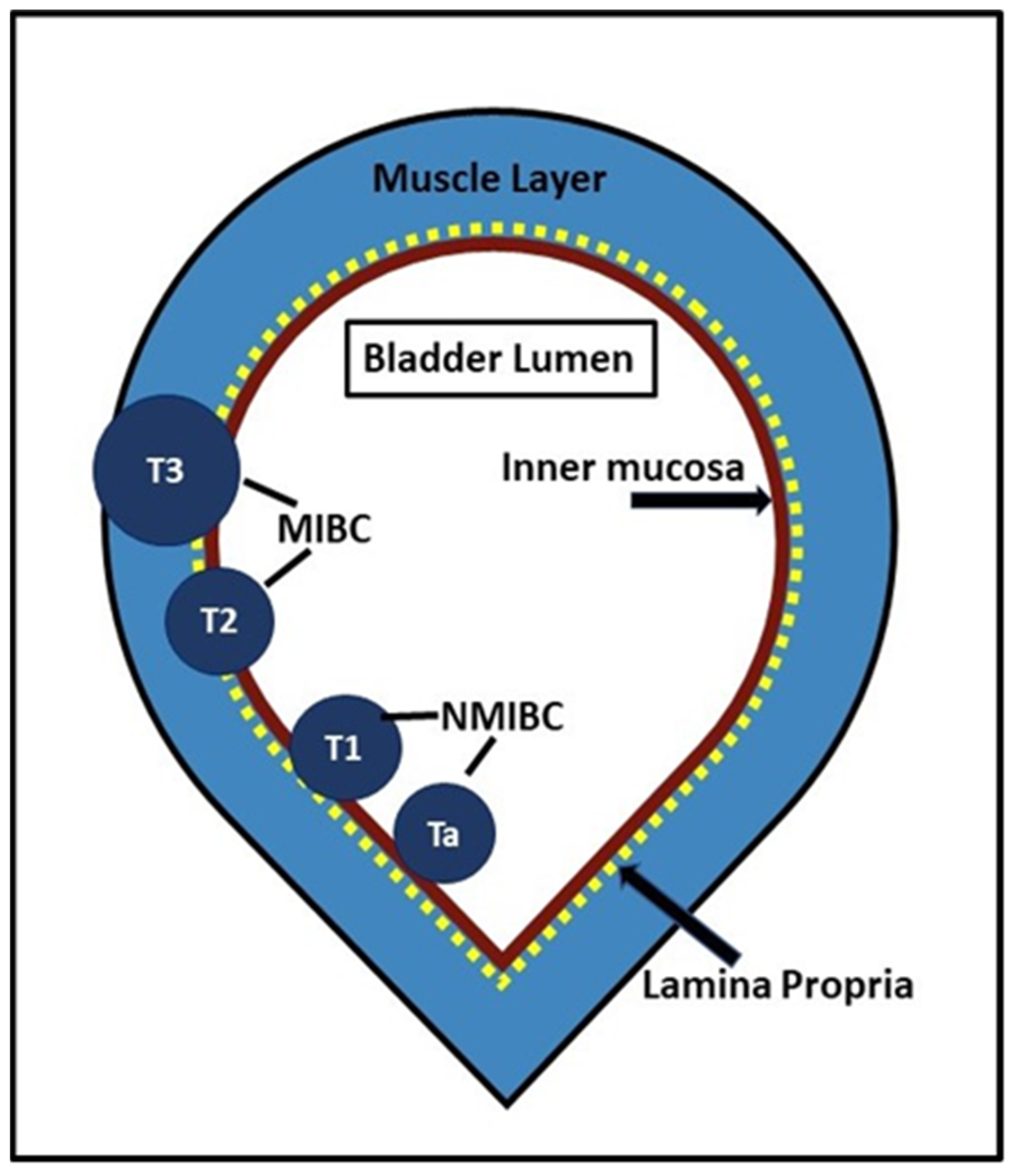
2. Current Challenges
2.1. Imaging Modalities
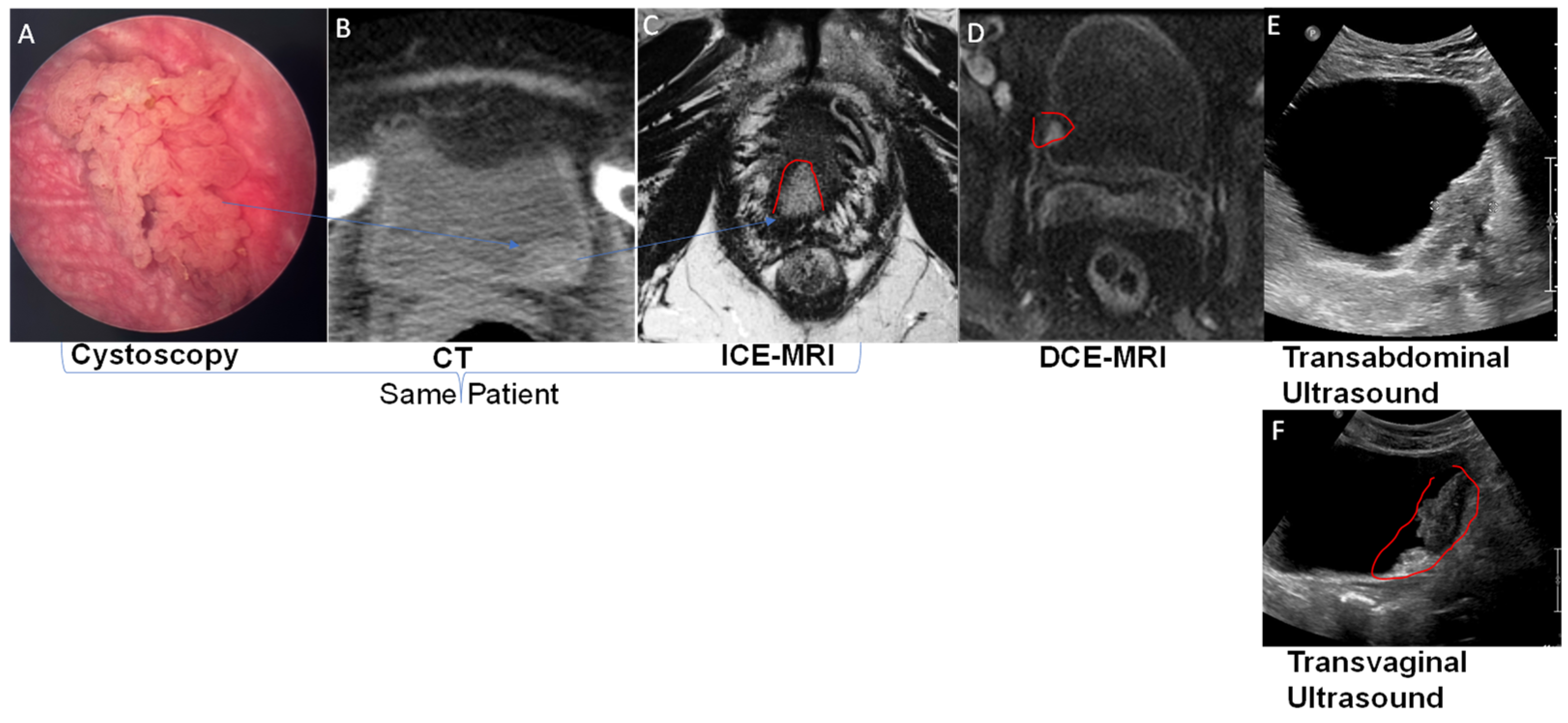
2.2. Magnetic Resonance Imaging (MRI)
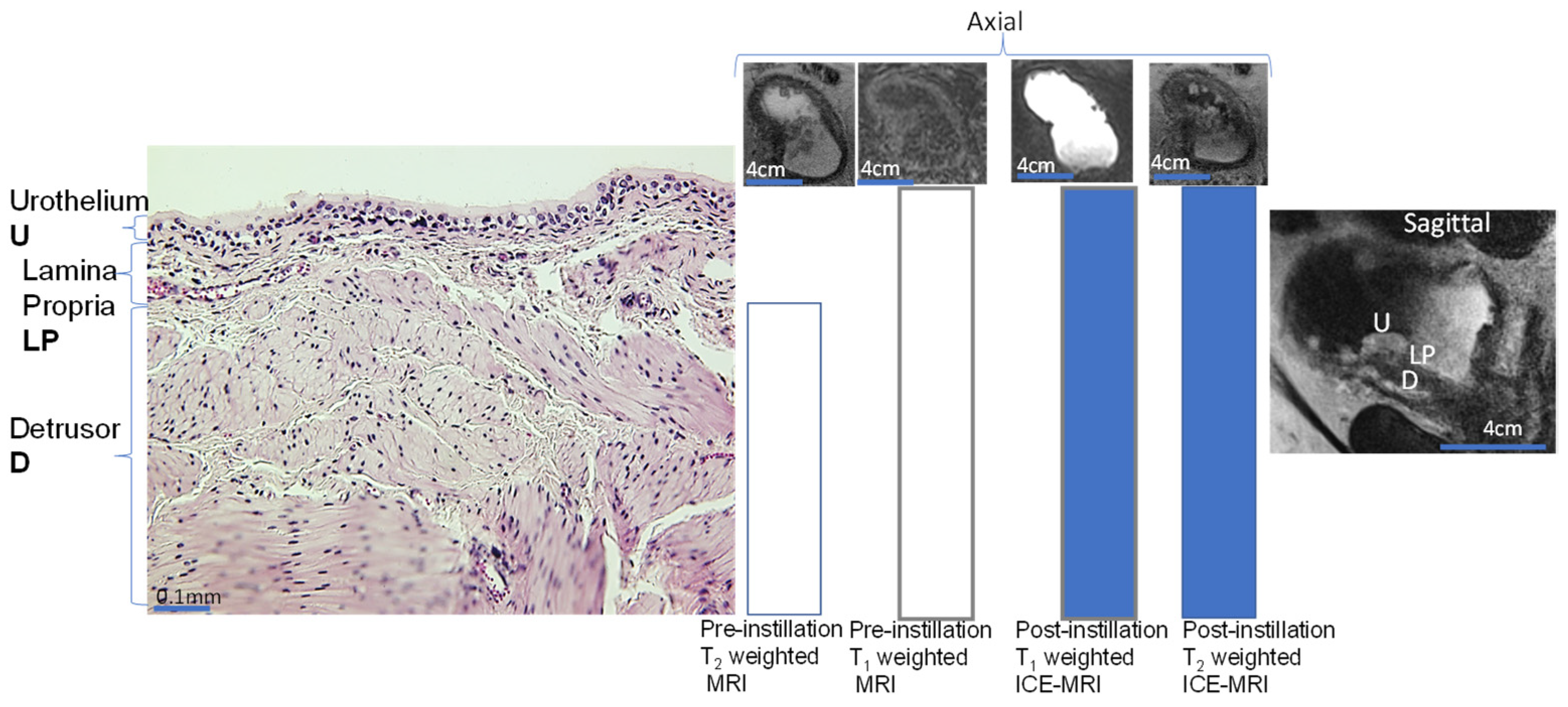
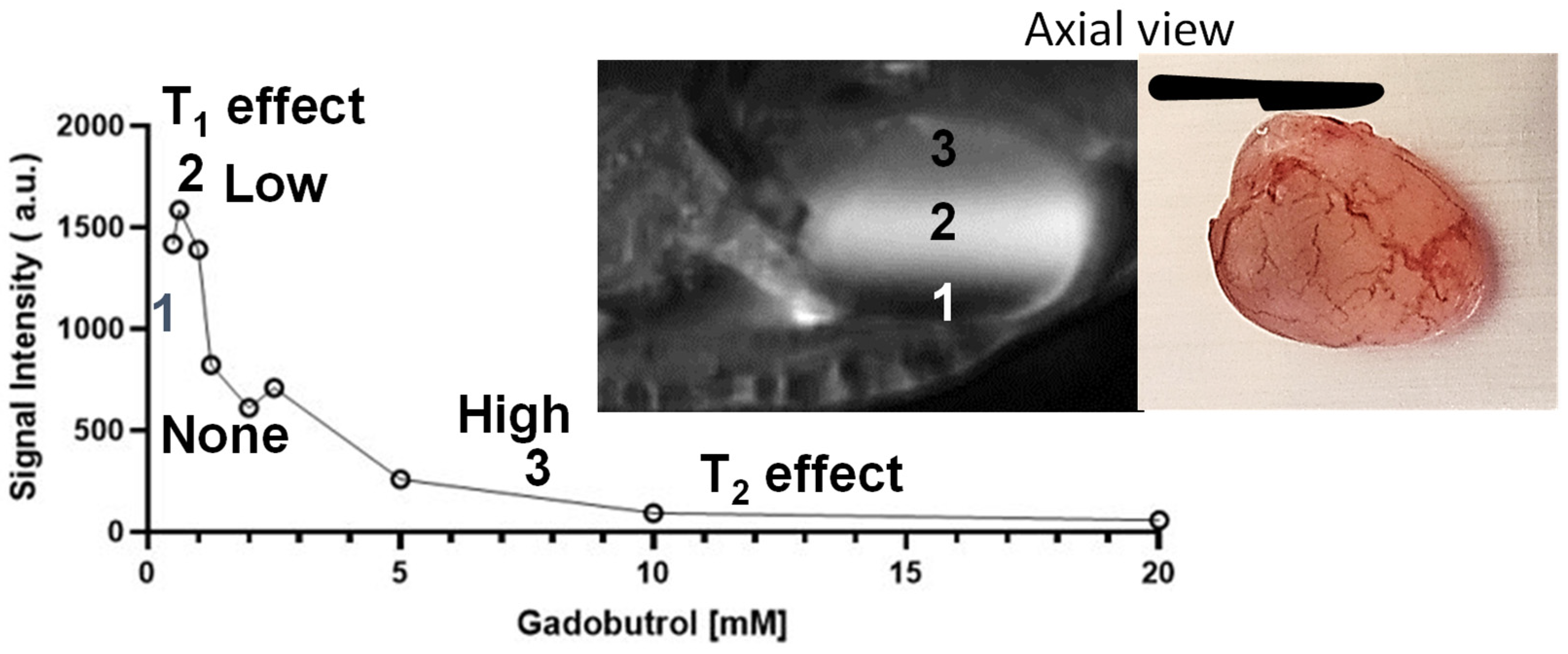
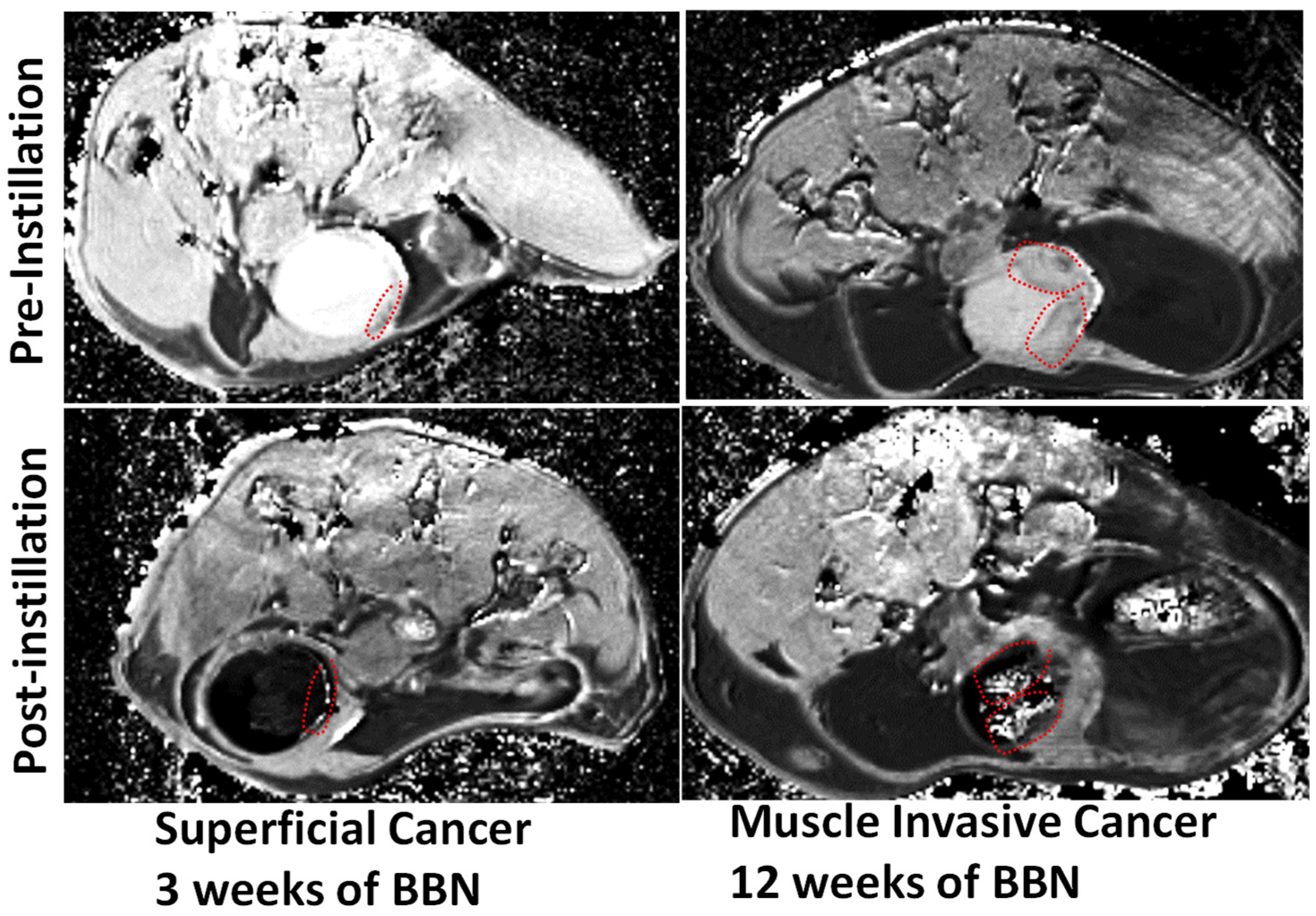
2.3. DCE-MRI
3. Intravesical Contrast-Enhanced MRI (ICE-MRI)
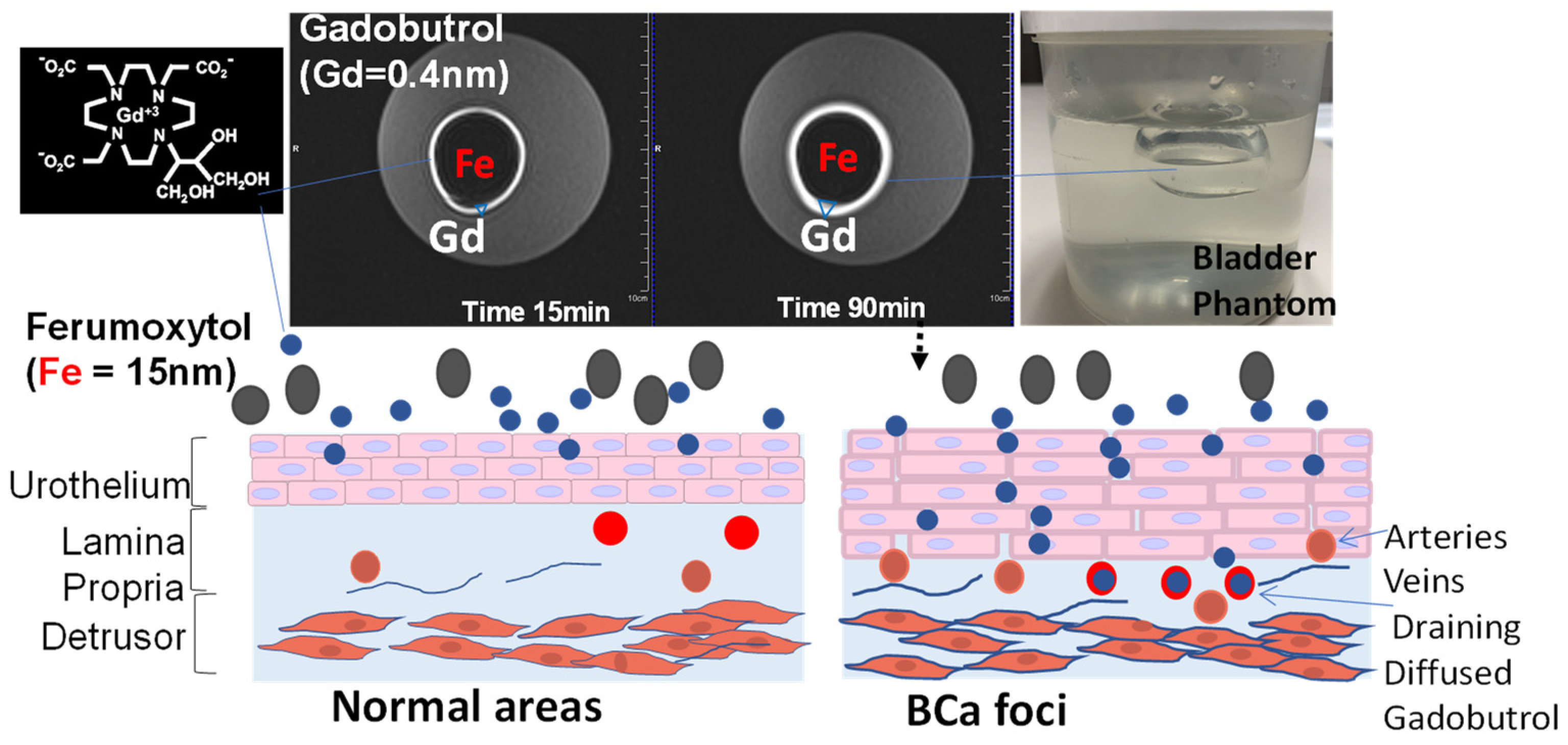
3.1. Past Attempts of Adding Negative Contrast to Bladder
3.2. Principle
3.3. Paracellular Path of Diffusion
3.4. Effect of Urinary Dilution on Image Contrast
3.5. Clinical Translation of ICE-MRI from 7T to 3T
References
- Kawada, T.; Yanagisawa, T.; Araki, M.; Pradere, B.; Shariat, S.F. Sequential intravesical gemcitabine and docetaxel therapy in patients with nonmuscle invasive bladder cancer: A systematic review and meta-analysis. Curr. Opin. Urol. 2023, 33, 211–218.
- Hugar, L.A.; Yabes, J.G.; Turner, R.M., 2nd; Fam, M.M.; Appleman, L.J.; Davies, B.J.; Jacobs, B.L. Rate and Determinants of Completing Neoadjuvant Chemotherapy in Medicare Beneficiaries With Bladder Cancer: A SEER-Medicare Analysis. Urology 2019, 124, 191–197.
- Ge, X.; Lan, Z.K.; Chen, J.; Zhu, S.Y. Effectiveness of contrast-enhanced ultrasound for detecting the staging and grading of bladder cancer: A systematic review and meta-analysis. Med. Ultrason. 2021, 23, 29–35.
- Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Jiang, Y.H.; Lee, C.L.; Yu, W.R.; Kuo, H.C. Possible Association between Bladder Wall Morphological Changes on Computed Tomography and Bladder-Centered Interstitial Cystitis/Bladder Pain Syndrome. Biomedicines 2021, 9, 1306.
- Husband, J.E. Staging bladder cancer. Clin. Radiol. 1992, 46, 153–159.
- Husband, J.E.; Olliff, J.F.; Williams, M.P.; Heron, C.W.; Cherryman, G.R. Bladder cancer: Staging with CT and MR imaging. Radiology 1989, 173, 435–440.
- Setty, B.N.; Holalkere, N.S.; Sahani, D.V.; Uppot, R.N.; Harisinghani, M.; Blake, M.A. State-of-the-art cross-sectional imaging in bladder cancer. Curr. Probl. Diagn. Radiol. 2007, 36, 83–96.
- Yaman, O.; Baltaci, S.; Arikan, N.; Yilmaz, E.; Gogus, O. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: Comparison with final pathological stage in invasive bladder carcinoma. Br. J. Urol. 1996, 78, 197–200.
- Wang, Y.; Liu, J.; Yang, X.; Liu, Y.; Liu, Y.; Li, Y.; Sun, L.; Yang, X.; Niu, H. Bacillus Calmette-Guerin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. OncoTargets Ther. 2018, 11, 2891–2899.
- Roudnicky, F.; Poyet, C.; Buser, L.; Saba, K.; Wild, P.; Otto, V.I.; Detmar, M. Characterization of Tumor Blood Vasculature Expression of Human Invasive Bladder Cancer by Laser Capture Microdissection and Transcriptional Profiling. Am. J. Pathol. 2020, 190, 1960–1970.
- Singh, R.; Saleemi, A.; Walsh, K.; Popert, R.; O’Brien, T. Near misses in bladder cancer—An airline safety approach to urology. Ann. R. Coll. Surg. Engl. 2003, 85, 378–381.
- Caglic, I.; Panebianco, V.; Vargas, H.A.; Bura, V.; Woo, S.; Pecoraro, M.; Cipollari, S.; Sala, E.; Barrett, T. MRI of Bladder Cancer: Local and Nodal Staging. J. Magn. Reson. Imaging 2020, 52, 649–667.
- Zhou, Y.; Abel, G.A.; Hamilton, W.; Singh, H.; Walter, F.M.; Lyratzopoulos, G. Imaging activity possibly signalling missed diagnostic opportunities in bladder and kidney cancer: A longitudinal data-linkage study using primary care electronic health records. Cancer Epidemiol. 2020, 66, 101703.
- Rabie, E.; Faeghi, F.; Izadpanahi, M.H.; Dayani, M.A. Role of Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Staging of Bladder Cancer. J. Clin. Diagn. Res. 2016, 10, TC01–TC05.
- Wu, L.M.; Chen, X.X.; Xu, J.R.; Zhang, X.F.; Suo, S.T.; Yao, Q.Y.; Fan, Y.; Hu, J. Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperative T staging of urinary bladder cancer: A large-scale, multiobserver prospective study on 3.0-T MRI. Acad. Radiol. 2013, 20, 939–946.
- Connell, M.; Dhir, R.; Moon, C.H.; Biatta, S.; Tarin, T.; Maranchie, J.; Tyagi, P. Discrimination of cystitis cystica from bladder cancer by intravesical contrast-enhanced magnetic resonance imaging (ICE-MRI). Can. Urol. Assoc. J. 2022, 16, S158.
- Steward, M.C.; Seo, Y.; Rawlings, J.M.; Case, R.M. Water permeability of acinar cell membranes in the isolated perfused rabbit mandibular salivary gland. J. Physiol. 1990, 431, 571–583.
- Dickie, B.R.; Banerji, A.; Kershaw, L.E.; McPartlin, A.; Choudhury, A.; West, C.M.; Rose, C.J. Improved accuracy and precision of tracer kinetic parameters by joint fitting to variable flip angle and dynamic contrast enhanced MRI data. Magn. Reson. Med. 2016, 76, 1270–1281.
- Kanazawa, Y.; Miyati, T.; Sato, O. Hemodynamic analysis of bladder tumors using T1-dynamic contrast-enhanced fast spin-echo MRI. Eur. J. Radiol. 2012, 81, 1682–1687.
- Singh, N.; Zabbarova, I.; Ikeda, Y.; Maranchie, J.; Chermansky, C.; Foley, L.; Hitchens, T.K.; Yoshimura, N.; Kanai, A.; Kaufman, J.; et al. Virtual measurements of paracellular permeability and chronic inflammation via color coded pixel-wise T1 mapping. Am. J. Physiol. Ren. Physiol. 2020, 319, F506–F514.
- Saito, T.; Hitchens, T.K.; Foley, L.M.; Singh, N.; Mizoguchi, S.; Kurobe, M.; Gotoh, D.; Ogawa, T.; Minagawa, T.; Ishizuka, O.; et al. Functional and histologic imaging of urinary bladder wall after exposure to psychological stress and protamine sulfate. Sci. Rep. 2021, 11, 19440.
- Parikh, N.; Ream, J.M.; Zhang, H.C.; Block, K.T.; Chandarana, H.; Rosenkrantz, A.B. Performance of simultaneous high temporal resolution quantitative perfusion imaging of bladder tumors and conventional multi-phase urography using a novel free-breathing continuously acquired radial compressed-sensing MRI sequence. Magn. Reson. Imaging 2016, 34, 694–698.
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306.
- Murata, N.; Gonzalez-Cuyar, L.F.; Murata, K.; Fligner, C.; Dills, R.; Hippe, D.; Maravilla, K.R. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Investig. Radiol. 2016, 51, 447–453.
- Tyagi, P.; Janicki, J.; Moon, C.H.; Kaufman, J.; Chermansky, C. Novel contrast mixture achieves contrast resolution of human bladder wall suitable for T1 mapping: Applications in interstitial cystitis and beyond. Int. Urol. Nephrol. 2018, 50, 401–409.
- Tyagi, P.; Janicki, J.J.; Hitchens, T.K.; Foley, L.M.; Kashyap, M.; Yoshhimura, N.; Kaufman, J. Novel Contrast Mixture Improves Bladder Wall Contrast For Visualizing Bladder Injury. Am. J. Physiol. Ren. Physiol. 2017, 313, F155–F162.
- Sparenberg, A.; Hamm, B.; Hammerer, P.; Samberger, V.; Wolf, K.J. . Rofo 1991, 155, 117–122.
- Gao, X.; Au, J.L.; Badalament, R.A.; Wientjes, M.G. Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin. Cancer Res. 1998, 4, 139–143.
- Horikawa, Y.; Sugano, K.; Shigyo, M.; Yamamoto, H.; Nakazono, M.; Fujimoto, H.; Kanai, Y.; Hirohashi, S.; Kakizoe, T.; Habuchi, T.; et al. Hypermethylation of an E-cadherin (CDH1) promoter region in high grade transitional cell carcinoma of the bladder comprising carcinoma in situ. J. Urol. 2003, 169, 1541–1545.
- Golijanin, J.; Amin, A.; Moshnikova, A.; Brito, J.M.; Tran, T.Y.; Adochite, R.C.; Andreev, G.O.; Crawford, T.; Engelman, D.M.; Andreev, O.A.; et al. Targeted imaging of urothelium carcinoma in human bladders by an ICG pHLIP peptide ex vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 11829–11834.
- Lee, Y.K.; Jhang, J.F.; Jiang, Y.H.; Hsu, Y.H.; Ho, H.C.; Kuo, H.C. Difference in electron microscopic findings among interstitial cystitis/bladder pain syndrome with distinct clinical and cystoscopic characteristics. Sci. Rep. 2021, 11, 17258.
- Eldrup, J.; Thorup, J.; Nielsen, S.L.; Hald, T.; Hainau, B. Permeability and ultrastructure of human bladder epithelium. Br. J. Urol. 1983, 55, 488–492.
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398.
- Kodzwa, R. ACR Manual on Contrast Media: 2018 Updates. Radiol. Technol. 2019, 91, 97–100.
- Ganguly, A.; Foley, L.; Hitchens, T.K.; Maranchie, J.; Ikeda, Y.; Zabbarova, I.; Kanai, A.; Yoshimura, N.; Tyagi, P. Virtual Monitoring of Bladder Cancer Progression In Mice With Intravesical Contrast Enhanced Magnetic Resonance Imaging (ICE-MRI). J. Urol. 2023, 209, e183.
- Wientjes, M.G.; Badalament, R.A.; Wang, R.C.; Hassan, F.; Au, J.L. Penetration of mitomycin C in human bladder. Cancer Res. 1993, 53, 3314–3320.
- Pan, Y.; Volkmer, J.P.; Mach, K.E.; Rouse, R.V.; Liu, J.J.; Sahoo, D.; Chang, T.C.; Metzner, T.J.; Kang, L.; van de Rijn, M.; et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med. 2014, 6, 260ra148.
- Davis, R.M.; Kiss, B.; Trivedi, D.R.; Metzner, T.J.; Liao, J.C.; Gambhir, S.S. Surface-Enhanced Raman Scattering Nanoparticles for Multiplexed Imaging of Bladder Cancer Tissue Permeability and Molecular Phenotype. ACS Nano 2018, 12, 9669–9679.
- Boireau, S.; Buchert, M.; Samuel, M.S.; Pannequin, J.; Ryan, J.L.; Choquet, A.; Chapuis, H.; Rebillard, X.; Avances, C.; Ernst, M.; et al. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis 2007, 28, 246–258.
- Bartolozzi, C.; Caramella, D.; Zampa, V.; Olmastroni, M.; Innocenti, P.; Menchi, I.; Lapini, A.; Amorosi, A. MR imaging with STIR technique and air insufflation for local staging of bladder neoplasms. Acta Radiol. 1992, 33, 577–581.
- Beyersdorff, D.; Taupitz, M.; Giessing, M.; Turk, I.; Schnorr, D.; Loening, S.; Hamm, B. . Rofo 2000, 172, 504–508.
- Kikuchi, E.; Xu, S.; Ohori, M.; Matei, C.; Lupu, M.; Menendez, S.; Koutcher, J.A.; Bochner, B.H. Detection and quantitative analysis of early stage orthotopic murine bladder tumor using in vivo magnetic resonance imaging. J. Urol. 2003, 170, 1375–1378.
- Lee, S.K.; Chang, Y.; Park, N.H.; Kim, Y.H.; Woo, S. Magnetic resonance voiding cystography in the diagnosis of vesicoureteral reflux: Comparative study with voiding cystourethrography. J. Magn. Reson. Imaging 2005, 21, 406–414.
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Investig. Radiol. 2015, 50, 330–338.
- Knobloch, G.; Colgan, T.; Wiens, C.N.; Wang, X.; Schubert, T.; Hernando, D.; Sharma, S.D.; Reeder, S.B. Relaxivity of Ferumoxytol at 1.5 T and 3.0 T. Investig. Radiol. 2018, 53, 257–263.
- Finn, J.P.; Nguyen, K.L.; Han, F.; Zhou, Z.; Salusky, I.; Ayad, I.; Hu, P. Cardiovascular MRI with ferumoxytol. Clin. Radiol. 2016, 71, 796–806.
- Gupta, T.; Virmani, S.; Neidt, T.M.; Szolc-Kowalska, B.; Sato, K.T.; Ryu, R.K.; Lewandowski, R.J.; Gates, V.L.; Woloschak, G.E.; Salem, R.; et al. MR tracking of iron-labeled glass radioembolization microspheres during transcatheter delivery to rabbit VX2 liver tumors: Feasibility study. Radiology 2008, 249, 845–854.
- Mantovani, L.F.; Santos, F.P.S.; Perini, G.F.; Nascimento, C.M.B.; Silva, L.P.; Wroclawski, C.K.; Esposito, B.P.; Ribeiro, M.S.S.; Velloso, E.; Nomura, C.H.; et al. Hepatic and cardiac and iron overload detected by T2* magnetic resonance (MRI) in patients with myelodisplastic syndrome: A cross-sectional study. Leuk. Res. 2019, 76, 53–57.
- Chin, J.; Kadhim, S.; Garcia, B.; Kim, Y.S.; Karlik, S. Magnetic resonance imaging for detecting and treatment monitoring of orthotopic murine bladder tumor implants. J. Urol. 1991, 145, 1297–1301.
- Tyagi, P.; Moon, C.H.; Janicki, J.; Kaufman, J.; Chancellor, M.; Yoshimura, N.; Chermansky, C. Recent advances in imaging and understanding interstitial cystitis. F1000Research 2018, 7, 1771.
- Fellows, G.J. Permeability of normal and diseased human bladder epithelium. Proc. R. Soc. Med. 1972, 65, 299–300.
- Sonn, G.A.; Jones, S.N.; Tarin, T.V.; Du, C.B.; Mach, K.E.; Jensen, K.C.; Liao, J.C. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J. Urol. 2009, 182, 1299–1305.
- Fukui, I.; Yokokawa, M.; Mitani, G.; Ohwada, F.; Wakui, M.; Washizuka, M.; Tohma, T.; Igarashi, K.; Yamada, T. In vivo staining test with methylene blue for bladder cancer. J. Urol. 1983, 130, 252–255.
- Gill, W.B.; Huffman, J.L.; Lyon, E.S.; Bagley, D.H.; Schoenberg, H.W.; Straus, F.H., 2nd. Selective surface staining of bladder tumors by intravesical methylene blue with enhanced endoscopic identification. Cancer 1984, 53, 2724–2727.
- Gill, W.B.; Strauss, F.H. In vivo mapping of bladder cancer (chromocystoscopy for in vivo detection of neoplastic urothelial surfaces). Urology 1984, 23, 63–66.
- Malamitsi, J.; Zorzos, J.; Varvarigou, A.D.; Archimandritis, S.; Dassiou, C.; Sivolapenko, G.; Skarlos, D.V.; Serefoglou, A.; Lykourinas, M.; Proukakis, C. Immunotargeting of urothelial cell carcinoma with intravesically administered Tc-99m labeled HMFG1 monoclonal antibody. Cell Biophys. 1994, 24-25, 75–81.
- Malamitsi, J.; Zorzos, J.; Varvarigou, A.D.; Archimandritis, S.; Dassiou, C.; Skarlos, D.V.; Dimitriou, P.; Likourinas, M.; Zizi, A.; Proukakis, C. Immunolocalization of transitional cell carcinoma of the bladder with intravesically administered technetium-99m labelled HMFG1 monoclonal antibody. Eur. J. Nucl. Med. 1995, 22, 25–31.
- Syrigos, K.N.; Khawaja, M.; Krausz, T.; Williams, G.; Epenetos, A.A. Intravesical administration of radiolabelled tumour-associated monoclonal antibody in bladder cancer. Acta Oncol. 1999, 38, 379–382.
- Bamias, A.; Keane, P.; Krausz, T.; Williams, G.; Epenetos, A.A. Intravesical administration of radiolabeled antitumor monoclonal antibody in bladder carcinoma. Cancer Res. 1991, 51, 724–728.
- Koenig, S.H.; Spiller, M.; Brown, R.D., 3rd; Wolf, G.L. Relaxation of water protons in the intra- and extracellular regions of blood containing Gd(DTPA). Magn. Reson. Med. 1986, 3, 791–795.
- Elsen, S.; Lerut, E.; Van Cleynenbreugel, B.; van der Aa, F.; van Poppel, H.; de Witte, P.A. Biodistribution of Evans blue in an orthotopic AY-27 rat bladder urothelial cell carcinoma model: Implication for the improved diagnosis of non-muscle-invasive bladder cancer (NMIBC) using dye-guided white-light cystoscopy. BJU Int. 2015, 116, 468–477.
- Elsen, S.; Lerut, E.; Van Der Aa, F.; Van Cleynenbreugel, B.; Van Poppel, H.; De Witte, P. Evans blue-mediated white-light detection of non-muscle-invasive bladder cancer: A preclinical feasibility and safety study using a rat bladder urothelial cell carcinoma model. Mol. Clin. Oncol. 2016, 5, 678–688.
- Eichel, L.; Scheidweiler, K.; Kost, J.; Shojaie, J.; Schwarz, E.; Messing, E.; Wood, R. Assessment of murine bladder permeability with fluorescein: Validation with cyclophosphamide and protamine. Urology 2001, 58, 113–118.
- Carattino, M.D.; Prakasam, H.S.; Ruiz, W.G.; Clayton, D.R.; McGuire, M.; Gallo, L.I.; Apodaca, G. Bladder filling and voiding affect umbrella cell tight junction organization and function. Am. J. Physiol. Ren. Physiol. 2013, 305, F1158–F1168.
- Holmes, D.L.; Stellwagen, N.C. Estimation of polyacrylamide gel pore size from Ferguson plots of linear DNA fragments. II. Comparison of gels with different crosslinker concentrations, added agarose and added linear polyacrylamide. Electrophoresis 1991, 12, 612–619.
- Tyagi, P.; Moon, C.; Singh, N.; Connell, M.; Maranchie, J.; Chermansky, C.; Yoshimura, N.; Kaufman, J. High Resolution 3D T1-Mapping of Pig Bladder Wall by Intravesical Contrast Enhanced MRI At 3T. J. Urol. 2021, 206, e390.
- Tyagi, P.; Moon, C.; Singh, N.; Connell, M.; Maranchie, J.; Chermansky, C.; Yoshimura, N.; Kaufman, J. Probing the bladder wall diffusion of instilled gadobutrol by MRI. J. Urol. 2021, 206, e32.





