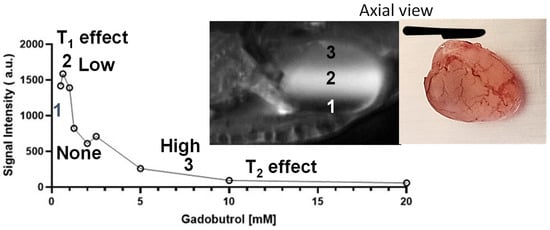
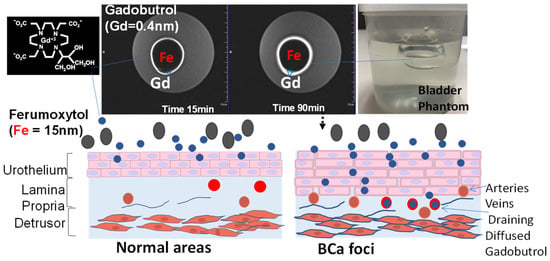
MRI of a bladder phantom. The principle of Stokesian diffusion dictates passive, isotropic, time-dependent diffusion of Gadobutrol (0.4 nm) into >20 times bigger pores of polyacrylamide gel over 90 min (indicated by bigger blue∇ in the right image). The bright ring increasing from 15 to 90 min, which depicts paracellular Gd diffusion, is thrown into sharp relief by the dark cavity mimicking the luminal retention of a larger-sized Ferumoxytol (Fe 15 nm), with its larger magnetic moment decaying the Gadobutrol signal within the lumen.
Figure 2 and
Figure 5 illustrate that ICE-MRI
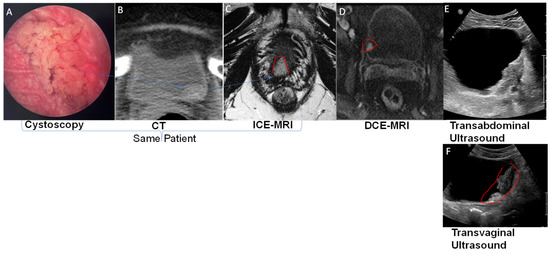
[25][26][53,54] provides stable positive contrast in rodent and human bladders, and the period of artifact-free visualization can be extended nearly 10-fold compared to DCE-MRI
[22][36]. On the other hand, the reduced bioavailability of Gadobutrol dose 1 mmol (seven times lower than the recommended intravenous dose)
[14][25][27][27,53,55] instilled into bladder eliminates the inherent risks of heavy metal toxicity and allergic reaction associated with GBCA injection
[33][34][61,62]. Preclinical findings of a dark lumen adjacent to a bright bladder wall
[20][21][26][30,31,54], generated by ICE-MRI at 7T
[26][35][54,63], and a 9.4T animal scanner
[21][30] were reproduced in the T2-weighted turbo spin echo images acquired at clinical scanner 3T (
Figure 2C and
Figure 3).
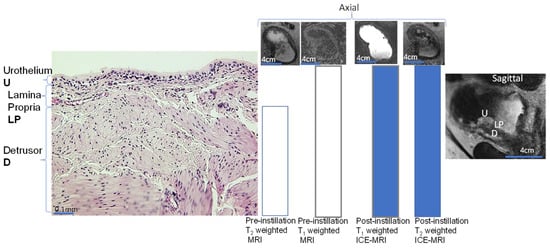
The graded decline in the signal intensity across bladder wall tissue layers (
Figure 3 and
Figure 5) manifests the logarithmic decline of diffusing Gadobutrol concentration
[20][31] from the mucosa to deeper tissue layers. The logarithmic decline in diffused Gadobutrol concentration
[20][31] stems from homeostatic venous clearance of any instilled drug reaching mucosa,
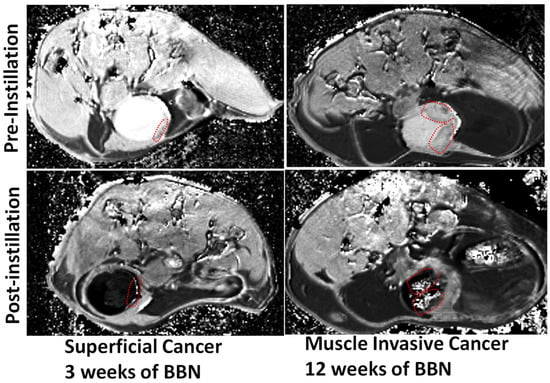
[36][64]. Angiogenesis of the bladder tumor
[35][63] augments the venous drainage of diffused Gadobutrol to accentuate the concentration gradient which may accelerate the Fickian diffusion of instilled Gadobutrol. As a result, the intensity of enhancement descends from aggressive cancer lesion > indolent cancer lesion > non-cancerous bladder wall (
Figure 5). Cancerous lesions
[37][38][65,66] on the luminal surface of the bladder are characterized by a disrupted tight junction barrier
[39][67], and tumoritropic infiltration
[35][63] of GBCA generates localized enhancement (
Figure 2C).
3.1. Past Attempts of Adding Negative Contrast to Bladder
Since pseudolayering in the bladder lumen with DCE-MRI results from a lack of uniform contrast in the lumen, several group resorted to insufflation air
[40][68] or instillation of Ferumoxytol
[41][69] to ensure uniformity of contrast in the human bladder lumen during DCE-MRI. While Ferumoxytol instillation alone was directly tried in humans, air insufflation was also tried in a mouse bladder together with GBCA instillation
[42][70]. However, both approaches failed to offer any advantage in improving the accuracy of BCa staging. As extensively reported by several groups
[27][43][55,71], bladder instillation of just GBCA alone without the inclusion of a negative MR contrast (i.e., a hypo-intensity signal) is unable to achieve a clear visualization of bladder wall, which is a prerequisite for BCa staging.
3.2. Principle
The innovation of ICE-MRI capitalizes on the inverse relationship between the diffusion rate and the Stokes–Einstein radius of instilled drugs. Stokesian diffusion dictates the paracellular diffusion of smaller Gadobutrol (with a Stokes–Einstein radius of 0.4 nm and a molecular weight of 604.7 Daltons), whereas the diffusion of larger-sized Ferumoxytol (with a Stokes–Einstein radius of 15 nm and a molecular weight of 731 kiloDaltons) is retarded. Gadobutrol
[44][72] and Ferumoxytol
[45][46][47][48][73,74,75,76] also differ in their magnetic moment for tumorotropic infiltration of Gadobutrol
[42][49][70,77] to cause tumor enhancement
[20][21][30,31], while luminal retention of Ferumoxytol darkens the bladder lumen
[35][63] (
Figure 5 and
Figure 6), as the large magnetic moment of iron
[45][46][47][48][73,74,75,76] induces local magnetic field inhomogeneities to dephase proton spins, causing signal decay within the bladder lumen (
Figure 6)
[25][26][50][53,54,78].
3.3. Paracellular Path of Diffusion
ICE-MRI is predicated on the perturbed tight junctions
[39][51][67,85] of bladder tumors relative to the normal areas that are shown to cause tumoritropic infiltration of small molecular weight drugs/dyes: such as mitomycin
[36][64], fluorescein
[52][86], methylene blue
[53][54][55][87,88,89]. However, diffusion of high molecular weight radiolabeled probes
[56][57][58][59][79,80,81,82] around tight junctions is slowed in accordance with the principle of Stokesian diffusion. Instilled GBCA is unlikely to enter umbrella cells as the transcellular permeability of umbrella cells is restricted, and GBCA is unable to enter even red blood cells upon injection
[60][90]. The perturbed tight junctions
[39][51][67,85] of cancer foci are known to compromise the urothelial barrier, which accentuates the passive diffusion of instilled GBCA in both rodent (
Figure 5)
[35][37][42][49][63,65,70,77] and human bladder (
Figure 2 and
Figure 3)
[27][55], analogous to the diffusion of instilled polar dyes in preclinical
[61][62][63][91,92,93] and clinical
[52][53][54][55][86,87,88,89] studies. The differential signal enhancement of cancer foci by ICE-MRI replicates the results obtained with other radiation-free approaches
[30][52][58,86].
3.4. Effect of Urinary Dilution on Image Contrast
Given that the physical gap of the apico-lateral tight junction
[61][62][64][91,92,94] (
Figure 2 and
Figure 3) can be partly mimicked by the pore size of 12% polyacrylamide gel
[65][95], scholarsrelied on that equivalence to study the paracellular diffusion
[20][31] of Gadobutrol without the confounding influence (
Figure 6) of bladder distension and bladder perfusion
[66][67][84,96]. A spherical bladder-shaped cavity was molded with 12% polyacrylamide gel poured into a plastic container, which was wrapped by a 4-channel flexible receiver coil, for image acquisition using a multi-echo spoiled-gradient echo pulse sequence in 3T scanner (Siemens, BioGraph)
Figure 6.
3.5. Clinical Translation of ICE-MRI from 7T to 3T
The ten-fold lower thickness of rodent bladder wall (~0.5 mm) compared to human bladder wall (~5 mm) requires a proportionally higher signal-to-noise ratio of ≥7T for imaging mouse bladder cancer (
Figure 5)
[35][63]. The clinical translation of ICE-MRI tackled differences in field strength and pulse sequences from spin echo at a higher field of 7T to gradient echo (FLASH) at 3T for human subjects (
Figure 2 and
Figure 3) by adjusting the concentration of instilled Gadobutrol and Ferumoxytol. Accordingly, to limit signal dephasing in bladder wall with the use of gradient echo for T1 weighted imaging, scholarslowered the Ferumoxytol concentration
[66][84] from our past study
[25][53] by raising the Gadobutrol concentration
[66][84].