Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anatoliy V Popov | -- | 1652 | 2023-05-31 18:41:46 | | | |
| 2 | Jason Zhu | Meta information modification | 1652 | 2023-06-01 03:22:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Menchikov, L.G.; Popov, A.V. Organic Germanium Compounds. Encyclopedia. Available online: https://encyclopedia.pub/entry/45080 (accessed on 07 February 2026).
Menchikov LG, Popov AV. Organic Germanium Compounds. Encyclopedia. Available at: https://encyclopedia.pub/entry/45080. Accessed February 07, 2026.
Menchikov, Leonid G., Anatoliy V. Popov. "Organic Germanium Compounds" Encyclopedia, https://encyclopedia.pub/entry/45080 (accessed February 07, 2026).
Menchikov, L.G., & Popov, A.V. (2023, May 31). Organic Germanium Compounds. In Encyclopedia. https://encyclopedia.pub/entry/45080
Menchikov, Leonid G. and Anatoliy V. Popov. "Organic Germanium Compounds." Encyclopedia. Web. 31 May, 2023.
Copy Citation
Germanium is an essential microelement, and its deficiency can result in numerous diseases, particularly oncogenic conditions. Consequently, water-soluble germanium compounds, including inorganic and coordination compounds, have attracted significant attention due to their biological activity.
germanium compounds
Ge-132
germatrane
biological activity
1. Germanium Sesquioxides
The most-studied organic germanium compound is bis(carboxy ethylgermanium) sesquioxide (Ge-132). Its synthesis is carried out by the addition of trichlorogermane (HgeCl3) to acrylic acid to produce 3-(trichlorogermyl)propanoic acid, followed by the hydrolysis thereof. In this reaction, the trichlorogermyl group Cl3Ge regiospecifically adds to the terminal carbon atom of the vinyl group of acrylic acids (Figure 1) [1][2][3].
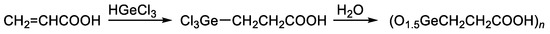
Figure 1. Synthesis of Ge-132.
Since the first synthesis of this compound was reported 55 years ago [2] the process of producing the original trichlorogermane has gone from a technically complex synthesis from elemental germanium [4] to the development of a simple and convenient method using germanium dioxide (GeO2), HCl and H3PO2 [5]. As a result, Ge-132 and other germanium sesquioxides are now readily available.
Regarding concerns over the alleged high toxicity of Ge-132 (see Section 2), its toxicity [6][7][8] and possible carcinogenicity [9][10] have been studied many times over the past 10 years. The results of the research once again confirmed the complete safety of Ge-132. The comprehensive study of the various biological activities of Ge-132 [11], including those that were previously known [12], particularly its antitumor [13][14][15][16][17][18][19] and immunomodulating [20][21][22][23] activities, also continued. In addition, Ge-132 is proposed as a treatment for a number of diseases: haemorrhagic and ischemic stroke [24][25], viral infections [20][22][26][27][28] (including COVID-19 [20]), various inflammatory diseases, particularly mastitis [29]. Next, this is suggested in the treatment of diabetes mellitus to reduce insulin resistance [30], and as an antioxidant for various disorders caused by oxidative stress [22][31][32][33][34] as well as in dermatological practice to heal skin wounds and protect the skin from reactive oxygen species [35][36]. Finally, Ge-132, together with hydroxyapatite, is proposed for the recovery and regeneration of mineralized tissues, particularly bone marrow [37]. The biological activity of Ge-132 is described in detail in the recently published monograph [38].
Structural studies of bis(carboxy ethylgermanium) sesquioxide have shown that, in solid form, it can exist in several polymeric forms (repagermanium RGe, propagermanium PGe and linear polymer GeSP) (Figure 2) [26]. The structure of the polymer affects the rapidity and completeness of its solubility in water and, as a consequence, its biological activity and dosage. When dissolved in water, it turns into a hydrated form-3-(trihydroxygermil)propanoic acid (THGPA). PGe possesses the best water solubility.
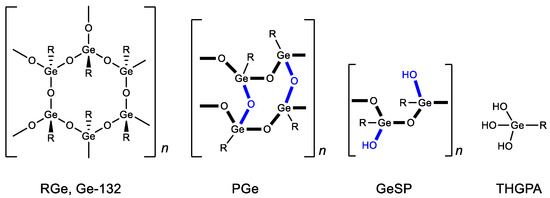
Figure 2. Forms of Ge-132. R = CH2CH2COOH.
As a result, there has recently been increased interest in Ge-132 in its most soluble form, PGe. For example, it is currently used in Japan to treat virus-hepatitis B [26]. Another direction in the study of Ge-132 biological activity is associated with the direct use of its hydrated form, THGPA. Thus, THGPA is shown to inhibit melanoma cell proliferation through phagocytosis [39]. Furthermore, it was revealed to have analgesic [40] and anti-inflammatory effects [41].
THGPA contains three hydroxy groups in its molecule, which can react with OH- groups of vital molecules. Such interactions may explain a number of physiological effects of Ge-132. Thus, to assess the possible mechanisms of this physiological activity, the interaction of THGPA with biologically active compounds such as adrenaline and ATP, which have vicinal diol functional groups, has been studied in detail. The interaction with these diols explains the numerous physiological functions of Ge-132 at low toxicities [21][41]. It was later found that, in solution, THGPA can form complexes with nucleotides or nucleosides containing cis-diol fragments [42]. At the same time, the ability of THGPA to form complexes with nucleotides depended on the number of phosphate groups present at the ribose residue. Interestingly, THGPA inhibits the enzymatic activity of adenosine deaminase (ADA) when using adenosine as a substrate [42].
Given the presence of several reaction centers in the Ge-132 molecule, chemical modification has been explored to increase biological activity and broaden its scope of application. Several Ge-132 derivatives have been synthesized, including those substituted on the carboxylic group, 3-alkylsubstituted, and those with substitutes on the germanium atom.
It was previously shown that the introduction of aromatic and heteroaromatic substituents (quinolin, anthraquinone and naphthalene) as an ester group in Ge-132 increased their antitumor activity compared to Ge-132 itself [12][43]. At the same time, the introduction of an alkyl replacement in propionic acid position 2 (R1 = Alk) significantly reduced antitumor activity (Figure 3) [12][43].
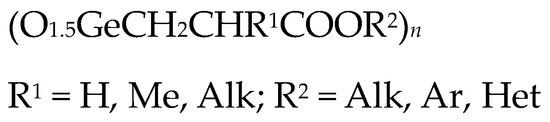
Figure 3. Derivatives of Ge-132.
Later esters with naphthalene and phenanthrene fragments, as well as N-arylamides with anthraquinone and dibenzofuran fragments, were synthesized (Figure 4) [44][45]. The resulting compounds had a stronger cytotoxic activity than Ge-132. The derivatives of methacrylic acid (R1 = Me) were therefore less active than similar derivatives of acrylic acid (R1 = H) [44][45]. These studies demonstrate possible means of Ge-132 modification to enhance its biological activity.
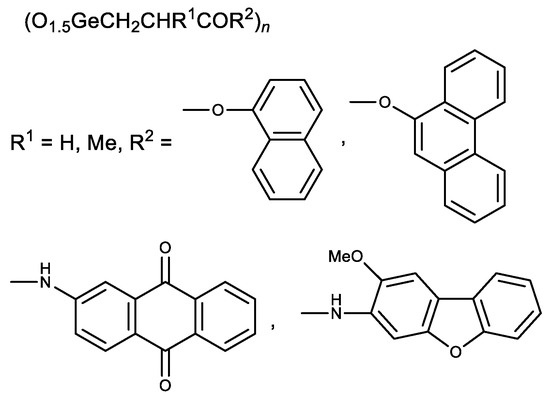
Figure 4. Aromatic derivatives of Ge-132.
In parallel with the derivatives of Ge-132, a germanium sesquioxide with resveratrol was synthesized (Figure 5) [46]. The antioxidant activity of the resulting compound was higher than that of Ge-132 and resveratrol separately, i.e., a synergistic effect was observed.
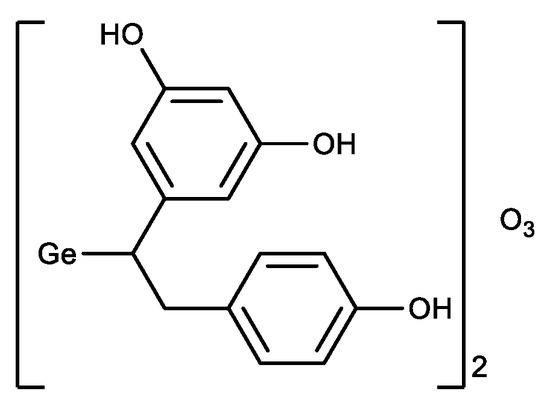
Figure 5. Germanium complex with resveratrol.
2. Germatranes, Germocanes
Germatranes (1) are another interesting class of biologically active germanium compounds, which are cyclic molecules stabilized by the hypervalent germanium atom (Figure 6) [47][48][49][50][51][52].
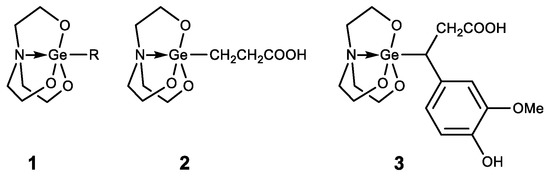
Figure 6. Germatranes.
Several compounds were identified as having high biological activity, including a peculiar hybrid of Ge-132 and germatrane-3-germatranyl substituted propionic acid (2) and its derivatives, which showed strong activity against various tumors [53][54][55]. Based on caffeic acid 3-germatranyl-3-(4-hydroxy-3-methoxyphenyl) propionic acid (3) was synthesized, which showed strong activity against cervical tumor U14 (in vitro and in vivo). This had inhibitory activity against cervical cancer cell line U14 with an IC50 as high as 48.57 mg/L (117.32 µM), whereas the degree of inhibition of the tumor growth is 64% in the animal experiment [56]. 2-aminoethoxy-substituted germatrane (1, R = OCH2CH2NH2) inhibits the activity of mononuclear alkaline phospholipase A2, and may serve for the development of new antisclerotic drugs to prevent lipid metabolism disorders [57]. In addition, this compound has a beneficial effect on the bioenergetic characteristics of mitochondria, increasing the efficiency of oxidative phosphorylation and increasing the oxidation rate of NAD-dependent substrates by mitochondria [58][59][60]. Germatranol (1, R = OH) reveals a similar activity; it also acts as an antioxidant and reduces the content of reactive oxygen species (ROS) in plant cells [61]. Germatranol contains a hydroxy group, which (like the hydrated form of Ge-132) can interact with functional groups in vital molecules. Thus, germatranol-hydrate interacts with simple amino acids (glycine, L-alanine, β-alanine, and L-valine), resulting in corresponding aminocarboxygermanates [62].
In addition to germatranes, their bicyclic analogues—germocanes (quasigermatranes, 4) and monocyclic analogues-hypogermatranes (5) have been synthesized, and their biological activity was found to be similar to that of germatranes (Figure 7) [50][63][64][65][66][67][68].
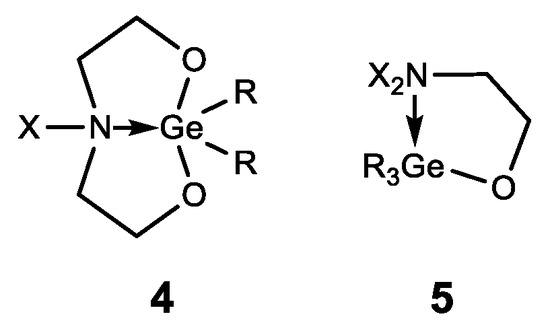
Figure 7. Germocanes and hypogermatranes.
The hypogermatranes 6 [69] and 7 [70] obtained in this way are molecules in which the ligands are coordinated to the germanium atom (Figure 8). These compounds exhibit antimicrobial activity against various strains of fungi and bacteria. Their pesticide activity against Corcyra Cephalonica is also established.
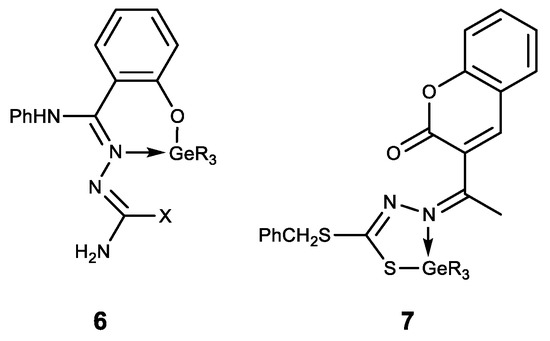
Figure 8. Hypogermatranes. R = Me, Ph; X = OH, SH.
Hypogermatranes 8, in which the ligands are coordinated with Ge (IV) via azomethine nitrogen atom and sulfur thiol/enol oxygen atom, are also known (Figure 9) [71][72]. These compounds have strong fungicidal and bactericidal properties. Furthermore, they are antioxidants and DNA splitters, whereas the compounds 8b showed strong antifertile activity [72].
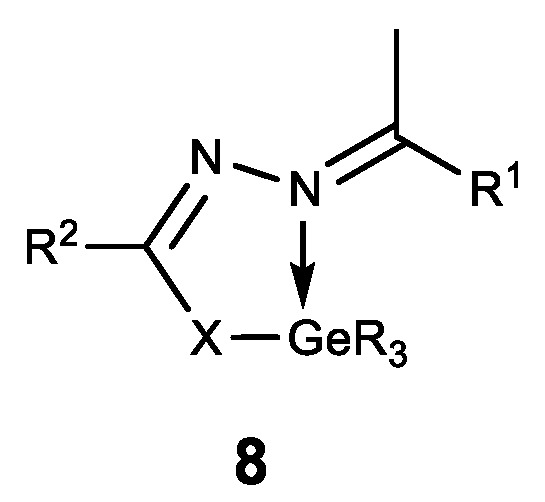
Figure 9. Hypogermatranes. R = Me, Ph; 8a: R1 = ferrocenyl, R2 = NH2; X = O, S; 8b: R1 = furan-2-yl, pyridine-2-yl, R2 = Py; X = O.
Finally, the first stable water-soluble germylene (a compound of divalent germanium) 9 with dipyrromethane ligand was described and its biological activity was studied (Figure 10) [73]. Compound 9 has been shown to have a comparable antiproliferative effect to cisplatin. These results form the basis for further biological research using germylenes, which are highly active compounds of low-valence germanium.
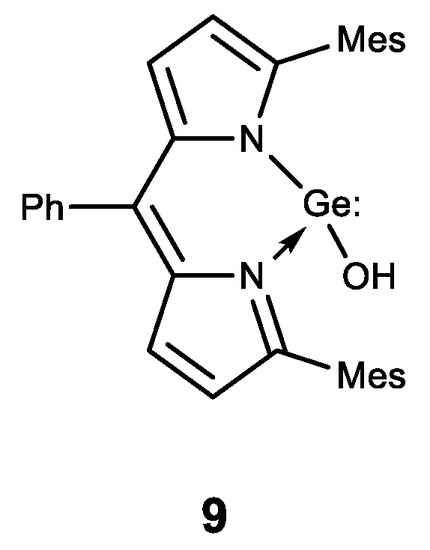
Figure 10. Stable water-soluble germylene.
3. Other Germanium Compounds
Among the compounds of other classes, germanium was introduced to compounds with known physiological activity. The obtained compounds had a synergistic effect. One of these compounds is ascorbic acid, where germanium was introduced as a substituent. Thus, an amide of trimethylgermylpropionic acid 10 was synthesized (Figure 11). It possesses high antioxidant properties and is proposed for the treatment of atopic dermatitis [74][75]. Similarly, a stable lipophilic ascorbic acid 11 derivative with high antioxidant activity was obtained (Figure 11) [32].
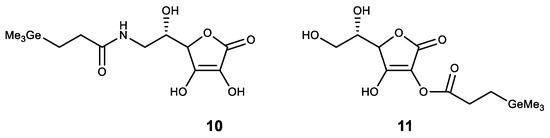
Figure 11. Germanium complexes with ascorbic acid.
A natural flavone crysine with a wide range of biological activity was also modified in this way. The resulting germanium complex with crysine (12) exhibits a synergistic effect as an antioxidant (Figure 12) [76].
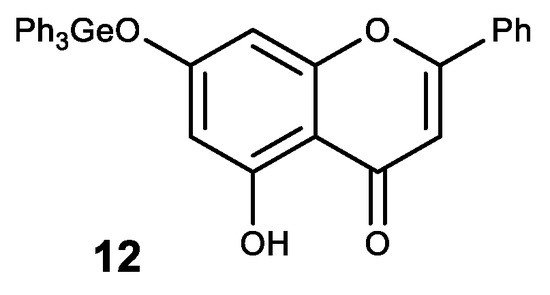
Figure 12. Germanium complex with crysine.
Complex 12 also showed high antitcancer activity. Thus, it has a significant inhibitory effect on the proliferation and growth of human cancer cell lines MCF-7, HepG2 and Colo205 with high selectivity between cancerous and normal cells [77][78]. An inhibitory effect on the proliferation of these cell lines is thought to occur through the induction of apoptosis via the ROS-dependent mitochondrial pathway [77][78].
Germanium was also introduced into dihydroartemisinin (DHA) as an analogue of Ge-132 (product of GeHCl3 addition to crotonic acid) (Figure 13) [79]. The resulting DHA-Ge complex 13 displays a synergistic effect of DHA and Ge-132, i.e., effectively inhibits the proliferation of HepG2 cells and can induce their apoptosis. Complex 13 is regarded as a promising antitumor agent [79].
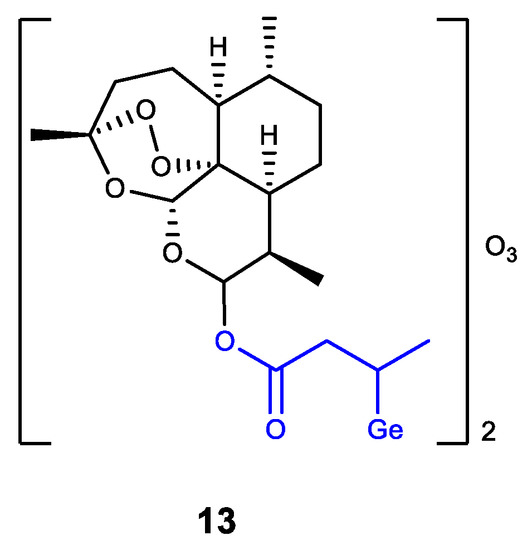
Figure 13. Germanium complex with dihydroartemisinin.
Steroids are another class of physiologically active compounds in which germanium was introduced as a substituent to position 16 [80][81][82]. The predicted biological activity of these and a number of other similar compounds was calculated by QSAR [83]. Antitumor, antiseborrheic and dermatological activities are the most characteristic predicted biological properties for these steroids.
Apart from the modification of natural compounds, GeR3 moeity is introduced to various heterocyclic derivatives. Thus, a number of germylsubstituted hetarylbenzimidazoles (14) was synthesized, and showed high cytotoxicity on the cell lines MG-22A, HT-1080 and NIH 3T3 (Figure 14) [84]. A similar series of germylsubstituted pyrane-3-carbonitriles (15) also showed high cytotoxicity and the inhibition of matrix metalloproteinase (Figure 14) [85]. The introduction of a germyl substituent in the heterocyclic position 5 (in furan or thiophene) was demonstrated to contribute to the emergence of cytotoxicity.
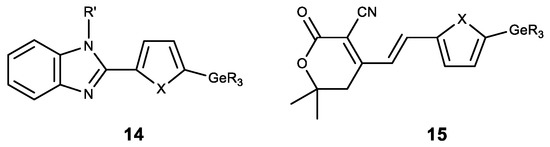
Figure 14. Germanium complexes with heterocycles. R = Me, Et; R’ = H, Me, Alk, CH2C≡CH; X = O, S.
References
- Kolesnikov, S.P. Research in Chemistry of Trihalogermans and Germanium Analogues of Dihalocarbenes; N.D. Zelinsky Institute of Organic Chemistry, Academy of Sciencies of USSR: Moscow, Russia, 1966. (In Russian)
- Mironov, V.F.; Berliner, E.M.; Gar, T.K. Reactions of trichlorogermane with acrylic acid and its derivatives. J. Gen. Chem. USSR Engl. Transl. 1967, 37, 911–912.
- Mironov, V.F.; Berliner, E.M.; Gar, T.K.; Rybakov, E.A. Reactions of Trichlorogermane with Unsaturated Carboxylic Acids. J. Gen. Chem. USSR Engl. Transl. 1968, 38, 2218.
- Nefedov, O.M.; Kolesnikov, S.P. Trichlorogermane etherates. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 1910.
- Shangguan, G.Q.; Zhang, S.G.; Ni, J.Z. Synthesis, structures and antitumor activities of beta-phenol ester propyl germanium sesquioxides. Chin. Chem. Lett. 1995, 6, 945–946.
- Keith, L.S.; Faroon, O.M.; Maples-Reynolds, N.; Fowler, B.A. Germanium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 799–816.
- Keith, L.S.; Maples-Reynolds, N. Germanium. In Handbook on the Toxicology of Metals. Volume II: Specific Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: London, UK; San Diego, CA, USA, 2022; pp. 289–316.
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Murbach, T.S.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Germanium Sesquioxide (Organic Germanium). J. Toxicol. 2020, 2020, 6275625.
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No carcinogenicity of poly-trans-(Ge-132): 26-week feeding study using rasH2 mice. Fundam. Toxicol. Sci. 2017, 4, 137–150.
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity study of poly-trans- (Ge-132) in F344 rats. Fundam. Toxicol. Sci. 2018, 5, 127–140.
- Rosenberg, E. Germanium-Containing Compounds, Current Knowledge and Applications. In Encyclopedia of Metalloproteins; Kretsinger, R., Uversky, V., Permyakov, E., Eds.; Springer: New York, NY, USA, 2013; pp. 847–855.
- Menchikov, L.G.; Ignatenko, M.A. Biological Activity of Organogermanium Compounds (A Review). Pharm. Chem. J. 2012, 46, 635–638.
- Ogwapit, S.M. Analysis of Ge-132 and development of a simple oral anticancer formulation. Biosci. Horiz. 2011, 4, 128–139.
- Vinodhini, J.; Kumar, D.S.R.S.; Sudha, S. Cytotoxic Effect of Carboxyethylgermanium Sesquioxide (Ge-132) on MCF-7 Human Breast Cancer Cell Line. Int. J. Curr. Sci. Res. IJCSR 2011, 2, 178–181.
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931.
- Masuda, T.; Tsuruda, Y.; Matsumoto, Y.; Uchida, H.; Nakayama, K.I.; Mimori, K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020, 111, 1039–1046.
- Vinodhini, J.; Sudha, S. Effect of bis-carboxy ethyl germanium sesquoxide on N-nitroso-N-methylurea-induced rat mammary carcinoma. Asian J. Pharm. Clin. Res. 2013, 6, 242–244.
- Ayodele, O.T. Anticancer activities of organogermanium compounds. Int. J. Progress. Sci. Technol. 2016, 3, 23–25.
- Shimada, Y.; Sato, K.; Tokuji, Y.; Nakamura, T. Nuclear magnetic resonance studies of the interactions between the organic germanium compound Ge-132 and saccharides. Carbohydr. Res. 2015, 407, 10–15.
- Narokha, V.; Nizhenkovska, I.; Kuznetsova, O. Potential of germanium-based compounds in coronavirus infection. Acta Pharm. 2022, 72, 245–258.
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246.
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge-132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753.
- Nakamura, T.; Takeda, T.; Tokuji, Y. The Oral Intake of Organic Germanium, Ge-132, Elevates α-Tocopherol Levels in the Plasma and Modulates Hepatic Gene Expression Profiles to Promote Immune Activation in Mice. Int. J. Vitam. Nutr. Res. 2014, 84, 0183–0195.
- Guo, F.; Xu, D.; Lin, Y.; Wang, G.; Wang, F.; Gao, Q.; Wei, Q.; Lei, S. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. 2020, 34, 1872–1884.
- He, S.; Liu, R.; Li, B.; Huang, L.; Fan, W.; Tembachako, C.R.; Zheng, X.; Xiong, X.; Miyata, M.; Xu, B.; et al. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019, 125, 99–110.
- Mizuno, N.; Nishibori, E.; Oka, M.; Jomori, T.; Takata, M.; Kumasaka, T. Structural Basis for Polymer Packing and Solvation Properties of the Organogermanium Crystalline Polymer Propagermanium and Its Derivatives. J. Pharm. Sci. 2015, 104, 2482–2488.
- Baidya, S.; Nishimoto, Y.; Sato, S.; Shimada, Y.; Sakurai, N.; Nonaka, H.; Noguchi, K.; Kido, M.; Tadano, S.; Ishikawa, K.; et al. Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses 2021, 13, 1674.
- Alimbarova, L.M.; Ambrosov, I.V.; Matelo, S.K.; Barinsky, I.F. Antiviral activity of the organic germanium complex with aciclovir against herpes simplex virus (Herpesviridae: Alphaherpesvirinae: Simplexvirus: Human alphaherpesvirus 1/2) in the in vitro and in vivo systems. Probl. Virol. 2021, 66, 368–382.
- Wang, Y.-S.; Teng, G.-Q.; Zhou, H.; Dong, C.-L. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol. Trace Elem. Res. 2020, 198, 617–626.
- Mulder, P.; van den Hoek, A.M.; Kleemann, R. The CCR2 Inhibitor Propagermanium Attenuates Diet-Induced Insulin Resistance, Adipose Tissue Inflammation and Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0169740.
- Kim, E.; Hwang, S.-U.; Yoon, J.D.; Jeung, E.-B.; Lee, E.; Kim, D.Y.; Hyun, S.-H. Carboxyethylgermanium sesquioxide (Ge-132) treatment during in vitro culture protects fertilized porcine embryos against oxidative stress induced apoptosis. J. Reprod. Dev. 2017, 63, 581–590.
- Oh, C.; Li, M.; Kim, E.H.; Park, J.S.; Lee, J.C.; Ham, S.W. Antioxidant and Radical Scavenging Activities of Ascorbic Acid Derivatives Conjugated with Organogermanium. Bull. Korean Chem. Soc. 2010, 31, 3513–3514.
- Tezuka, T.; Higashino, A.; Akiba, M.; Nakamura, T. Organogermanium (Ge-132) Suppresses Activities of Stress Enzymes Responsible for Active Oxygen Species in Monkey Liver Preparation. Adv. Enzym. Res. 2017, 5, 13.
- Kim, E.; Jeon, Y.; Kim, D.Y.; Lee, E.; Hyun, S.-H. Antioxidative effect of carboxyethylgermanium sesquioxide (Ge-132) on IVM of porcine oocytes and subsequent embryonic development after parthenogenetic activation and IVF. Theriogenology 2015, 84, 226–236.
- Matsumoto, H.; Iwafuji, H.; Yamane, J.; Takeuchi, R.; Utsunomiya, T.; Fujii, A. Restorative effect of organic germanium compound (Ge-132) on dermal injury. Wound Med. 2016, 15, 6–10.
- Takeda, T.; Doiyama, S.; Azumi, J.; Shimada, Y.; Tokuji, Y.; Yamaguchi, H.; Nagata, K.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium suppresses cell death due to oxidative stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 13637.
- Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.-A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S.D. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993.
- Nakamura, T.; Shimada, Y.; Sato, K. Bioorganic and Medicinal Organogermanium Chemistry. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 839–865.
- Azumi, J.; Takeda, T.; Shimada, Y.; Zhuang, T.; Tokuji, Y.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells Via Phagocytosis. Int. J. Mol. Sci. 2023, 24, 1885.
- Sekiguchi, F.; Koike, N.; Shimada, Y.; Sugimoto, K.; Masuda, H.; Nakamura, T.; Yamaguchi, H.; Tanabe, G.; Marumoto, S.; Kasanami, Y.; et al. A hydrolysate of poly-trans- (Ge-132) suppresses Cav3.2-dependent pain by sequestering exogenous and endogenous sulfide. Redox Biol. 2023, 59, 102579.
- Azumi, J.; Shimada, Y.; Takeda, T.; Aso, H.; Nakamura, T. The Organogermanium Compound 3-(Trihydroxygermyl) Propanoic Acid (THGP) Suppresses Inflammasome Activation Via Complexation with ATP. Int. J. Mol. Sci. 2022, 23, 13364.
- Shimada, Y.; Sato, K.; Takeda, T.; Tokuji, Y. The Organogermanium Compound Ge-132 Interacts with Nucleic Acid Components and Inhibits the Catalysis of Adenosine Substrate by Adenosine Deaminase. Biol. Trace Elem. Res. 2018, 181, 164–172.
- Rosenberg, E. Germanium: Environmental occurrence, importance and speciation. Rev. Environ. Sci. Biotechnol. 2009, 8, 29–57.
- Ge, S.; Zhao, Y.; Sui, B.; Shangguan, G. Studies on the interaction of novel organogermanium sesquioxides with DNA. Chem. Res. Chin. Univ. 2015, 31, 31–37.
- Zhao, Y.; Zhang, L.; Sui, B.; Shangguan, G. Synthesis and cytotoxicity of novel organogermanium sesquioxides. J. Jining Med. Univ. 2017, 40, 14.
- Yao, S.; Jiang, J.; Yang, P.; Cai, J.-Y. Synthesis, characterization and antioxidant activity of a novel organogermanium sesquioxide with resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122.
- Chernyshev, E.A.; Knyazev, S.P.; Kirin, V.N.; Vasilev, I.M.; Alekseev, N.V. Structural features of silatranes and germatranes. Russ. J. Gen. Chem. 2004, 74, 58–65.
- Ignatyev, I.S.; Sundius, T.R.; Vrazhnov, D.V.; Kochina, T.A.; Voronkov, M.G. Bonding in germatranyl cation and germatranes. J. Organomet. Chem. 2007, 692, 5697–5700.
- Zabalov, M.; Karlov, S.; Zaitseva, G.; Lemenovskii, D. The molecular and electronic structure features of silatranes, germatranes, and their carbon analogs. Russ. Chem. Bull. 2006, 55, 464–476.
- Voronkov, M.G.; Korlyukov, A.A.; Samokhin, G.S.; Vrazhnov, D.V.; Kochina, T.A. Germatranes and their quasi and hypo analogs with highly electronegative substituent at the Ge atom. Russ. Chem. Bull. 2012, 61, 992–998.
- Karlov, S.S.; Zaitseva, G.S. Germatranes and their Analogs. Synthesis, Structure, and Reactivity. (Review). Chem. Heterocycl. Compd. 2001, 37, 1325–1357.
- Xu, M.-Y.; Xiao, B. Germatranes and carbagermatranes: (hetero)aryl and alkyl coupling partners in Pd-catalyzed cross-coupling reactions. Chem. Commun. 2021, 57, 11764–11775.
- Lukevics, E.; Germane, S.; Zidermane, A.; Dauvarte, A.; Kravchenko, I.M.; Trusule, M.; Mironov, V.F.; Gar, T.K.; Khromova, N.Y. Synthesis, neurotropic and antitumor activity of some germatranes, germasesquioxanes and their organotin analogs. Pharm. Chem. J. 1984, 18, 89–94.
- Kakimoto, N.; Sato, K.; Takada, T.; Akiba, M. Organogermanium compounds: Synthesis, structure, and properties of masked ethoxycarbonylgermanium sesquioxide (GE-132) and related compounds with one triethanolamine component. Heterocycles 1987, 26, 347–353.
- Song, X.; Yang, Z.; Su, G.; Xieqinglan. Synthesis, characterization and anticancer activity of some bis(germylpropionato-di-n-butyltin) oxides. Phosphorus Sulfur Silicon Relat. Elem. 1999, 150–151, 367–374.
- Ye, L.; Ou, X.; Peng, X.; Luo, Y. Synthesis of 3-(2, 8, 9-trioxa-5-aza-1-germatricyclo Undecane-1- yl)-3-(4-hydroxyl-3-methoxyphenyl)-propionic Acid and its Inhibitory Effect on the Cervical Tumor U14 in vitro and in vivo. Med. Chem. 2012, 8, 595–598.
- Zhigacheva, I.V.; Baryshok, V.P.; Rasulov, M.M.; Storozhenko, P.A. 2-(Germatran-1-yloxy)ethylamine as an inhibitor of the total activity of mononuclear alkaline phospholipase A2. Russ. Chem. Bull. 2021, 70, 444–448.
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetic characteristics of mitochondria under stress and action of organogermanium compounds. J. Nat. Sci. Sustain. Technol. 2015, 9, 439.
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetical characteristics of mitochondria. In Chemical and Biochemical Physics: A Systematic Approach to Experiments, Evaluation, and Modeling; Schiraldi, D., Zaikov, G.E., Eds.; Apple Academic Press: Oakville, ON, Canada, 2016; pp. 259–276.
- Zhigacheva, I.V.; Burlakova, E.B. Plant Growth and Development Regulators and their Effect on the Functional State of Mitochondria. In Chemistry and Technology of Plant Substances; Kutchin, A.V., Shishkina, L.N., Weisfeld, L.I., Eds.; Apple Academic Press: New York, NY, USA, 2017; pp. 243–278.
- Shigarova, A.M.; Grabelnych, O.I.; Baryshok, V.P.; Borovskii, G.B. Impossible mechanisms of germatranol influence on the thermal stability of wheat germs. Appl. Biochem. Microbiol. 2016, 52, 429–434.
- Ignatyev, I.S.; Lezov, D.V.; Kondratenko, Y.A.; Kochina, T.A. Interaction of simple amino acids (glycine, α-alanine, β-alanine and L-valine) with germatranol hydrate. J. Mol. Struct. 2022, 1253, 132245.
- Baukov, Y.I.; Tandura, S.N. Hypervalent Compounds of Organic Germanium, Tin and Lead Derivatives. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Rappoport, Z., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, pp. 963–1239.
- Selina, A.; Karlov, S.; Zaitseva, G. Metallocanes of group 14 elements. 1. Derivatives of silicon and germanium. (Review). Chem. Heterocycl. Compd. 2006, 42, 1518–1556.
- Lermontova, E.K.; Churakov, A.V.; Quan, M.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S. Synthesis, structure, and reactivity of germanium-containing derivatives of substituted diethanolamines. Russ. J. Inorg. Chem. 2009, 54, 211–218.
- de Velasco, D.O.-G.; Sánchez-Jiménez, R.; Hernández-Ortega, S.; Toscano, R.A.; García-Montalvo, V. Study on the transannular bond formation and hypercoordination in tin and germanium spirometallocanes. Polyhedron 2010, 29, 2435–2439.
- Shainyan, B.A. Structural and Conformational Aspects in the Chemistry of Heterocycles. Molecules 2020, 25, 3461.
- Lukevics, E.; Ignatovich, L. Comparative study of the biological activity of organosilicon and organogermanium compounds. Appl. Organomet. Chem. 1992, 6, 113–126.
- Dawara, L.; Singh, D.; Singh, R.V. Antimicrobial and pesticidal activity of some organogermanium (IV) complexes synthesized under microwave irradiation. Main Group Met. Chem. 2011, 34, 69–75.
- Dawara, L.; Fahmi, N.; Singh, R.V. Synthesis, characterization, antimicrobial, pesticidal and DNA cleavage activity of germanium (IV) derivatives of 3-(2-methyl-2, 3-dihydro-benzthiazo-2-yl)-chromen-2-one and N′--hydrazinecarbodithionic acid benzyl ester ligands. Main Group Met. Chem. 2011, 34, 139–146.
- Fahmi, N.; Khedar, R.; Singh, R.V. Green synthesis of new Ge(IV) complexes with bio-potent ligands and their antimicrobial, DNA cleavage, and antioxidant activities. Russ. J. Gen. Chem. 2016, 86, 958–964.
- Singh, N.; Watts, S.; Joshi, S.C.; Singh, R.V. Pesticidal and antifertility activities of triorganogermanium(IV) complexes synthesized using a green chemical approach. Appl. Organomet. Chem. 2013, 27, 269–276.
- Mahawar, P.; Wasson, M.K.; Sharma, M.K.; Jha, C.K.; Mukherjee, G.; Vivekanandan, P.; Nagendran, S. A Prelude to Biogermylene Chemistry. Angew. Chem. Int. Ed. 2020, 59, 21377–21381.
- Lim, D.H.; Li, M.; Seo, J.-A.; Lim, K.-M.; Ham, S.W. A novel organogermanium protected atopic dermatitis induced by oxazolone. Bioorg. Med. Chem. Lett. 2010, 20, 4032–4034.
- Lim, D.H.; Li, M.; Kim, E.-h.; Ham, S.W. Synthesis of Novel Organogermanium Derivative Conjugated with Vitamin C and Study of its Antioxidant Effects. Bull. Korean Chem. Soc. 2010, 31, 1839–1840.
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium (IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732.
- Yang, F.; Jin, H.; Pi, J.; Jiang, J.-H.; Liu, L.; Bai, H.-H.; Yang, P.-H.; Cai, J.-Y. Anti-tumor activity evaluation of novel chrysin–organogermanium(IV) complex in MCF-7 cells. Bioorg. Med. Chem. Lett. 2013, 23, 5544–5551.
- Yang, F.; Gong, L.; Jin, H.; Pi, J.; Bai, H.; Wang, H.; Cai, H.; Yang, P.; Cai, J. Chrysin–organogermanium (IV) complex induced Colo205 cell apoptosis-associated mitochondrial function and anti-angiogenesis. Scanning 2015, 37, 246–257.
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-h. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisinin-organogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297.
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 1. Hydrogermylation of conjugated steroid enones. Russ. Chem. Bull. 1998, 47, 180–182.
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 2. Synthesis of steroid germatranes. Russ. Chem. Bull. 1999, 48, 1185–1186.
- Karpenko, R.G.; Krylova, I.V.; Kamernitskii, A.V. Germylated steroids. 3. Synthesis of trialkylgermylated steroids. Russ. Chem. Bull. 2011, 60, 2100–2102.
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological Activities of Organometalloid (As, At, B, Ge, Si, Se, Te) Steroids. J. Appl. Pharm. Sci. Vol 2017, 7, 184–202.
- Ignatovich, L.; Starkova, O.; Romanovs, V.; Sleiksha, I.; Shestakova, I.; Popelis, J.; Lukevics, E. Novel trialkylsilyl(germyl)-substituted thienyl- and furylbenzimidazoles and their N-substituted derivatives—Synthesis, structure and cytotoxic activity. Comptes Rendus Chim. 2013, 16, 621–627.
- Ignatovich, L.; Spura, J.; Muravenko, V.; Belyakov, S.; Popelis, J.; Shestakova, I.; Domrachova, I.; Gulbe, A.; Rudevica, Z.; Leonchiks, A. Synthesis, structure and biological activity of new 6,6-dimethyl-2-oxo-4-vinyl-5,6-dihydro-2H-pyran-3-carbonitriles. Appl. Organomet. Chem. 2015, 29, 756–763.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
01 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




