Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Germanium is an essential microelement, and its deficiency can result in numerous diseases, particularly oncogenic conditions. Consequently, water-soluble germanium compounds, including inorganic and coordination compounds, have attracted significant attention due to their biological activity.
- germanium compounds
- Ge-132
- germatrane
- biological activity
1. Germanium Sesquioxides
The most-studied organic germanium compound is bis(carboxy ethylgermanium) sesquioxide (Ge-132). Its synthesis is carried out by the addition of trichlorogermane (HgeCl3) to acrylic acid to produce 3-(trichlorogermyl)propanoic acid, followed by the hydrolysis thereof. In this reaction, the trichlorogermyl group Cl3Ge regiospecifically adds to the terminal carbon atom of the vinyl group of acrylic acids (Figure 1) [64,68,69].
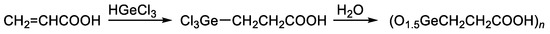
Figure 1. Synthesis of Ge-132.
Since the first synthesis of this compound was reported 55 years ago [68] the process of producing the original trichlorogermane has gone from a technically complex synthesis from elemental germanium [74] to the development of a simple and convenient method using germanium dioxide (GeO2), HCl and H3PO2 [75]. As a result, Ge-132 and other germanium sesquioxides are now readily available.
Regarding concerns over the alleged high toxicity of Ge-132 (see Section 2), its toxicity [56,57,58] and possible carcinogenicity [76,77] have been studied many times over the past 10 years. The results of the research once again confirmed the complete safety of Ge-132. The comprehensive study of the various biological activities of Ge-132 [8], including those that were previously known [36], particularly its antitumor [78,79,80,81,82,83,84] and immunomodulating [43,52,54,85] activities, also continued. In addition, Ge-132 is proposed as a treatment for a number of diseases: haemorrhagic and ischemic stroke [86,87], viral infections [43,54,88,89,90] (including COVID-19 [43]), various inflammatory diseases, particularly mastitis [4]. Next, this is suggested in the treatment of diabetes mellitus to reduce insulin resistance [91], and as an antioxidant for various disorders caused by oxidative stress [53,54,92,93,94] as well as in dermatological practice to heal skin wounds and protect the skin from reactive oxygen species [95,96]. Finally, Ge-132, together with hydroxyapatite, is proposed for the recovery and regeneration of mineralized tissues, particularly bone marrow [97]. The biological activity of Ge-132 is described in detail in the recently published monograph [71].
Structural studies of bis(carboxy ethylgermanium) sesquioxide have shown that, in solid form, it can exist in several polymeric forms (repagermanium RGe, propagermanium PGe and linear polymer GeSP) (Figure 2) [88]. The structure of the polymer affects the rapidity and completeness of its solubility in water and, as a consequence, its biological activity and dosage. When dissolved in water, it turns into a hydrated form-3-(trihydroxygermil)propanoic acid (THGPA). PGe possesses the best water solubility.
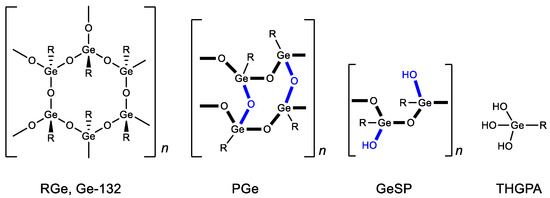
Figure 2. Forms of Ge-132. R = CH2CH2COOH.
As a result, there has recently been increased interest in Ge-132 in its most soluble form, PGe. For example, it is currently used in Japan to treat virus-hepatitis B [88]. Another direction in the study of Ge-132 biological activity is associated with the direct use of its hydrated form, THGPA. Thus, THGPA is shown to inhibit melanoma cell proliferation through phagocytosis [98]. Furthermore, it was revealed to have analgesic [99] and anti-inflammatory effects [100].
THGPA contains three hydroxy groups in its molecule, which can react with OH- groups of vital molecules. Such interactions may explain a number of physiological effects of Ge-132. Thus, to assess the possible mechanisms of this physiological activity, the interaction of THGPA with biologically active compounds such as adrenaline and ATP, which have vicinal diol functional groups, has been studied in detail. The interaction with these diols explains the numerous physiological functions of Ge-132 at low toxicities [52,100]. It was later found that, in solution, THGPA can form complexes with nucleotides or nucleosides containing cis-diol fragments [101]. At the same time, the ability of THGPA to form complexes with nucleotides depended on the number of phosphate groups present at the ribose residue. Interestingly, THGPA inhibits the enzymatic activity of adenosine deaminase (ADA) when using adenosine as a substrate [101].
Given the presence of several reaction centers in the Ge-132 molecule, chemical modification has been explored to increase biological activity and broaden its scope of application. Several Ge-132 derivatives have been synthesized, including those substituted on the carboxylic group, 3-alkylsubstituted, and those with substitutes on the germanium atom.
It was previously shown that the introduction of aromatic and heteroaromatic substituents (quinolin, anthraquinone and naphthalene) as an ester group in Ge-132 increased their antitumor activity compared to Ge-132 itself [24,36]. At the same time, the introduction of an alkyl replacement in propionic acid position 2 (R1 = Alk) significantly reduced antitumor activity (Figure 3) [24,36].
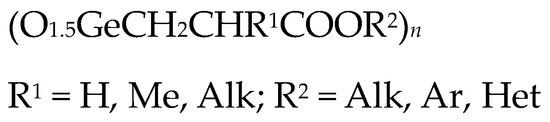
Figure 3. Derivatives of Ge-132.
Later esters with naphthalene and phenanthrene fragments, as well as N-arylamides with anthraquinone and dibenzofuran fragments, were synthesized (Figure 4) [102,103]. The resulting compounds had a stronger cytotoxic activity than Ge-132. The derivatives of methacrylic acid (R1 = Me) were therefore less active than similar derivatives of acrylic acid (R1 = H) [102,103]. These studies demonstrate possible means of Ge-132 modification to enhance its biological activity.
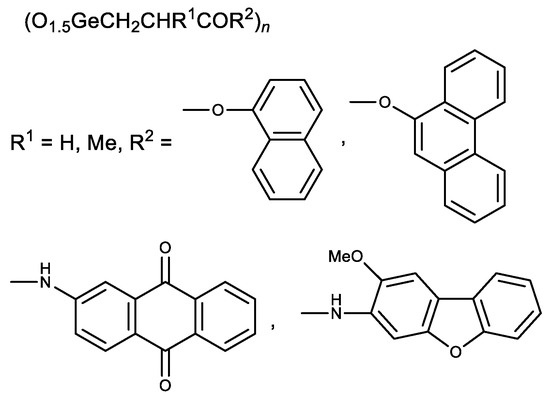
Figure 4. Aromatic derivatives of Ge-132.
In parallel with the derivatives of Ge-132, a germanium sesquioxide with resveratrol was synthesized (Figure 5) [104]. The antioxidant activity of the resulting compound was higher than that of Ge-132 and resveratrol separately, i.e., a synergistic effect was observed.
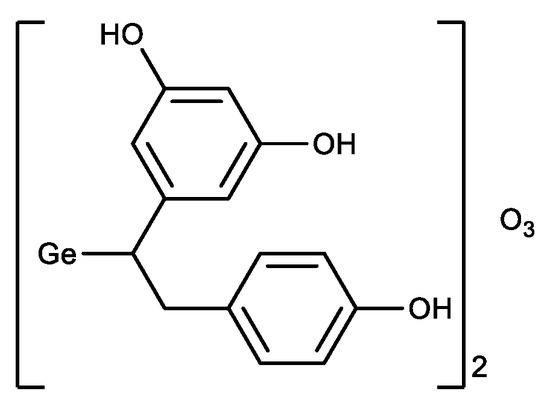
Figure 5. Germanium complex with resveratrol.
2. Germatranes, Germocanes
Germatranes (1) are another interesting class of biologically active germanium compounds, which are cyclic molecules stabilized by the hypervalent germanium atom (Figure 6) [105,106,107,108,109,110].
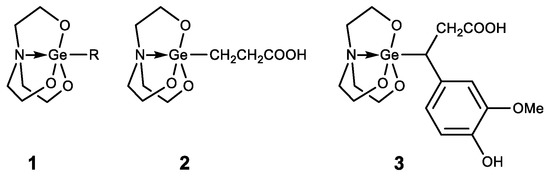
Figure 6. Germatranes.
Several compounds were identified as having high biological activity, including a peculiar hybrid of Ge-132 and germatrane-3-germatranyl substituted propionic acid (2) and its derivatives, which showed strong activity against various tumors [111,112,113]. Based on caffeic acid 3-germatranyl-3-(4-hydroxy-3-methoxyphenyl) propionic acid (3) was synthesized, which showed strong activity against cervical tumor U14 (in vitro and in vivo). This had inhibitory activity against cervical cancer cell line U14 with an IC50 as high as 48.57 mg/L (117.32 µM), whereas the degree of inhibition of the tumor growth is 64% in the animal experiment [114]. 2-aminoethoxy-substituted germatrane (1, R = OCH2CH2NH2) inhibits the activity of mononuclear alkaline phospholipase A2, and may serve for the development of new antisclerotic drugs to prevent lipid metabolism disorders [115]. In addition, this compound has a beneficial effect on the bioenergetic characteristics of mitochondria, increasing the efficiency of oxidative phosphorylation and increasing the oxidation rate of NAD-dependent substrates by mitochondria [116,117,118]. Germatranol (1, R = OH) reveals a similar activity; it also acts as an antioxidant and reduces the content of reactive oxygen species (ROS) in plant cells [119]. Germatranol contains a hydroxy group, which (like the hydrated form of Ge-132) can interact with functional groups in vital molecules. Thus, germatranol-hydrate interacts with simple amino acids (glycine, L-alanine, β-alanine, and L-valine), resulting in corresponding aminocarboxygermanates [120].
In addition to germatranes, their bicyclic analogues—germocanes (quasigermatranes, 4) and monocyclic analogues-hypogermatranes (5) have been synthesized, and their biological activity was found to be similar to that of germatranes (Figure 7) [108,121,122,123,124,125,126].
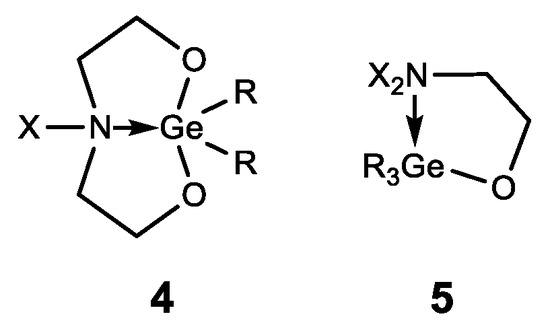
Figure 7. Germocanes and hypogermatranes.
The hypogermatranes 6 [127] and 7 [128] obtained in this way are molecules in which the ligands are coordinated to the germanium atom (Figure 8). These compounds exhibit antimicrobial activity against various strains of fungi and bacteria. Their pesticide activity against Corcyra Cephalonica is also established.
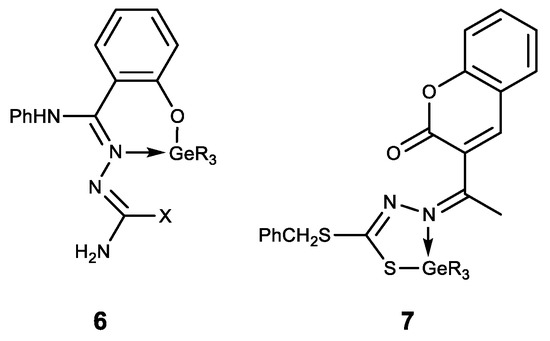
Figure 8. Hypogermatranes. R = Me, Ph; X = OH, SH.
Hypogermatranes 8, in which the ligands are coordinated with Ge (IV) via azomethine nitrogen atom and sulfur thiol/enol oxygen atom, are also known (Figure 9) [129,130]. These compounds have strong fungicidal and bactericidal properties. Furthermore, they are antioxidants and DNA splitters, whereas the compounds 8b showed strong antifertile activity [130].
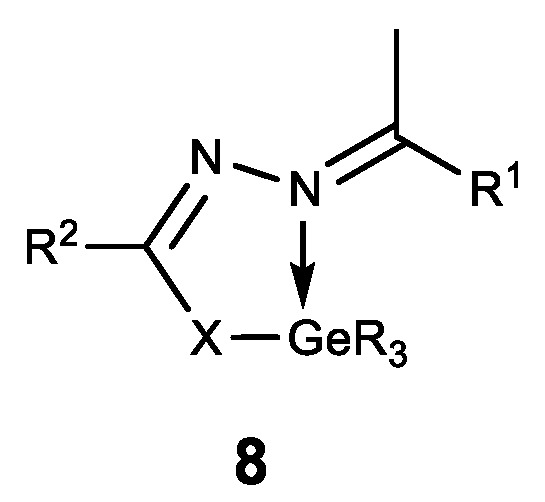
Figure 9. Hypogermatranes. R = Me, Ph; 8a: R1 = ferrocenyl, R2 = NH2; X = O, S; 8b: R1 = furan-2-yl, pyridine-2-yl, R2 = Py; X = O.
Finally, the first stable water-soluble germylene (a compound of divalent germanium) 9 with dipyrromethane ligand was described and its biological activity was studied (Figure 10) [131]. Compound 9 has been shown to have a comparable antiproliferative effect to cisplatin. These results form the basis for further biological research using germylenes, which are highly active compounds of low-valence germanium.
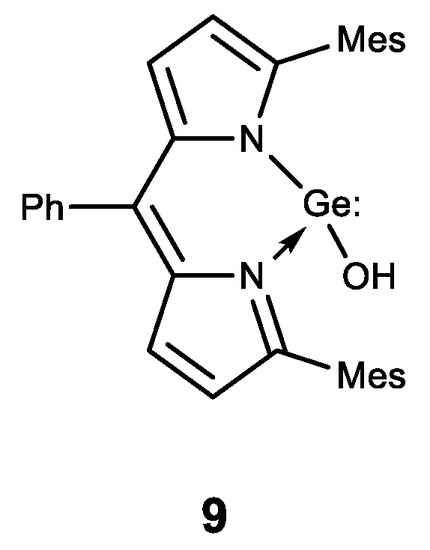
Figure 10. Stable water-soluble germylene.
3. Other Germanium Compounds
Among the compounds of other classes, germanium was introduced to compounds with known physiological activity. The obtained compounds had a synergistic effect. One of these compounds is ascorbic acid, where germanium was introduced as a substituent. Thus, an amide of trimethylgermylpropionic acid 10 was synthesized (Figure 11). It possesses high antioxidant properties and is proposed for the treatment of atopic dermatitis [132,133]. Similarly, a stable lipophilic ascorbic acid 11 derivative with high antioxidant activity was obtained (Figure 11) [92].
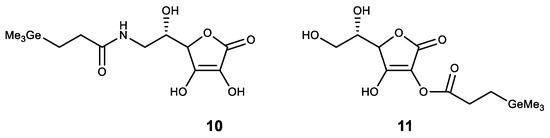
Figure 11. Germanium complexes with ascorbic acid.
A natural flavone crysine with a wide range of biological activity was also modified in this way. The resulting germanium complex with crysine (12) exhibits a synergistic effect as an antioxidant (Figure 12) [134].
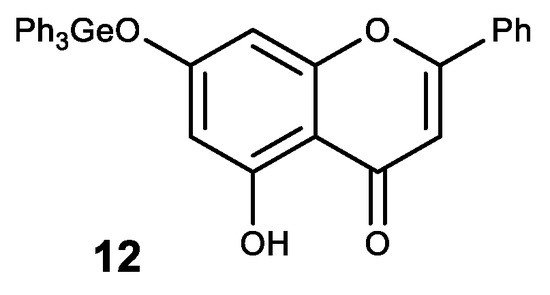
Figure 12. Germanium complex with crysine.
Complex 12 also showed high antitcancer activity. Thus, it has a significant inhibitory effect on the proliferation and growth of human cancer cell lines MCF-7, HepG2 and Colo205 with high selectivity between cancerous and normal cells [135,136]. An inhibitory effect on the proliferation of these cell lines is thought to occur through the induction of apoptosis via the ROS-dependent mitochondrial pathway [135,136].
Germanium was also introduced into dihydroartemisinin (DHA) as an analogue of Ge-132 (product of GeHCl3 addition to crotonic acid) (Figure 13) [137]. The resulting DHA-Ge complex 13 displays a synergistic effect of DHA and Ge-132, i.e., effectively inhibits the proliferation of HepG2 cells and can induce their apoptosis. Complex 13 is regarded as a promising antitumor agent [137].
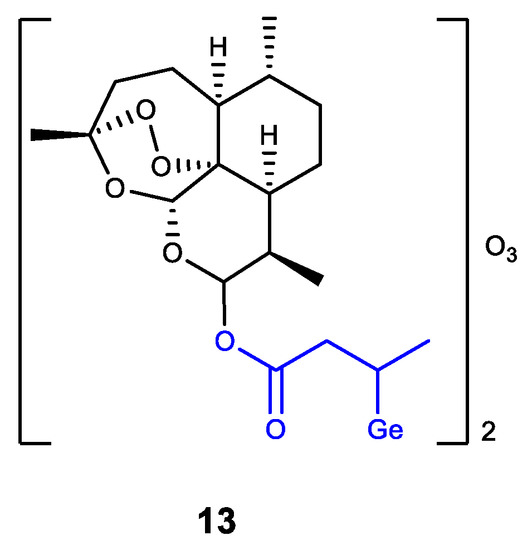
Figure 13. Germanium complex with dihydroartemisinin.
Steroids are another class of physiologically active compounds in which germanium was introduced as a substituent to position 16 [138,139,140]. The predicted biological activity of these and a number of other similar compounds was calculated by QSAR [141]. Antitumor, antiseborrheic and dermatological activities are the most characteristic predicted biological properties for these steroids.
Apart from the modification of natural compounds, GeR3 moeity is introduced to various heterocyclic derivatives. Thus, a number of germylsubstituted hetarylbenzimidazoles (14) was synthesized, and showed high cytotoxicity on the cell lines MG-22A, HT-1080 and NIH 3T3 (Figure 14) [142]. A similar series of germylsubstituted pyrane-3-carbonitriles (15) also showed high cytotoxicity and the inhibition of matrix metalloproteinase (Figure 14) [143]. The introduction of a germyl substituent in the heterocyclic position 5 (in furan or thiophene) was demonstrated to contribute to the emergence of cytotoxicity.
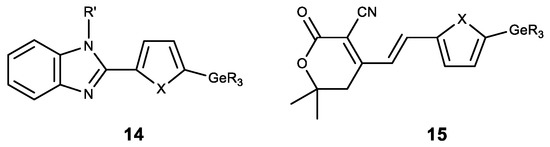
Figure 14. Germanium complexes with heterocycles. R = Me, Et; R’ = H, Me, Alk, CH2C≡CH; X = O, S.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11061535
This entry is offline, you can click here to edit this entry!
