Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Ferrante | -- | 3797 | 2023-05-31 16:41:02 | | | |
| 2 | Rita Xu | -2 word(s) | 3795 | 2023-06-01 03:19:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Leotta, L.; Toscano, S.; Ferrante, A.; Romano, D.; Francini, A. Woody Ornamental Plants in Mediterranean Climate. Encyclopedia. Available online: https://encyclopedia.pub/entry/45073 (accessed on 07 February 2026).
Leotta L, Toscano S, Ferrante A, Romano D, Francini A. Woody Ornamental Plants in Mediterranean Climate. Encyclopedia. Available at: https://encyclopedia.pub/entry/45073. Accessed February 07, 2026.
Leotta, Luca, Stefania Toscano, Antonio Ferrante, Daniela Romano, Alessandra Francini. "Woody Ornamental Plants in Mediterranean Climate" Encyclopedia, https://encyclopedia.pub/entry/45073 (accessed February 07, 2026).
Leotta, L., Toscano, S., Ferrante, A., Romano, D., & Francini, A. (2023, May 31). Woody Ornamental Plants in Mediterranean Climate. In Encyclopedia. https://encyclopedia.pub/entry/45073
Leotta, Luca, et al. "Woody Ornamental Plants in Mediterranean Climate." Encyclopedia. Web. 31 May, 2023.
Copy Citation
The native flora of different Mediterranean countries, often woody species, was widely recognized for its ornamental potential. The shrubs, in particular, are a typology of plants very widespread in the Mediterranean environment and constituent the ‘Macchia’, the typical vegetation of this ecosystem. These plant species could be used to improve the ornamental value of urban and peri-urban green areas. Since urban areas can suffer from low-quality soil and limited resources, the selection of plants must be carefully considered. The most commonly used plants should have adequate tolerance to abiotic stress.
urban environment
shrubs
green areas
landscape
abiotic stress
1. Introduction
Woody plants, trees or shrubs, represent the most common plants in many natural and semi-natural environments [1]. Almost all these plants have characteristics, such as being perennial or the same structure of ramifications, that allow their use for ornamental purposes [2]. All ‘woody ornamental plants’ can be used in gardens and landscaping, thanks to the presence of flowers, more or less showy, of different colours, the colour and morphology of leaves, and the shape of the plant (height, shape, and width) [2]. The species of woody ornamental plants belong to numerous botanical families and genera; within a single species, there are numerous cultivars, expressing enormous variability [3]. An indication of the wide biological diversity of woody plants used for horticultural purposes is the “List of Names of Woody Plants” by Naktuinbouw, which contains the ‘preferred botanical names and common synonyms and trade names of almost 45,000 woody nursery plants’ [4]. The new edition (2021–2025) contains more than 8200 new names of woody plants. Shrubs, in particular, are a category of woody plants widespread above all in the Mediterranean Basin, where they form the so-called in Italian ‘Macchia’, i.e., the vegetation typical of this environment.
The diffusion of this plant typology in the Mediterranean regions is justified by its adaptability to stressful climatic conditions, characterized by hot summers and low rainfall, which determine a long and droughty summer [5]. Climatic conditions of the Mediterranean environment are also found in four other regions of the Earth (California, Chile, South Africa, and some areas of Australia). The frequency of abiotic stresses, and in particular water and saline stresses due to the poor quality of the water, has selected the plants of all these environments, leading to the convergence of the morphophysiological traits of the various species, determining that all the plant communities of the Mediterranean climate are dominated by sclerophyllous evergreen shrubs. In the Mediterranean regions, water stress and poor water quality (high salt content) are among the main problems hindering the use of ornamental plants. Global climate change will certainly accentuate problems associated with water deficits and salt levels, especially in urban areas [6]. The possibility of the use of native Mediterranean shrub species can be a solution for drought [7] and saline stress [8]. It is of interest to increase the sustainability of the landscape. Native Mediterranean shrubs are able to adapt to conditions of accentuated drought, which is one of the most important factors influencing plant survival and species distribution [9]. Many woody ornamental plants and native Mediterranean shrubs are particularly suitable to use in landscape planning. These plants, in addition to their high aesthetic value, are characterized by wide biodiversity. Indeed, the Mediterranean Basin is one of the Earth’s areas with the greatest biodiversity, as it hosts 10% of the world’s higher plants in an area that is just 1.6% of the Earth’s surface [10]. Of the approximately 25,000 species recorded in the Mediterranean area, half are endemic to the region [11]. Hotspots represent about 22% of the total area of the Mediterranean Basin and host about 5500 restricted endemic species [12].
Thanks to their particular morpho-physiological characteristics, woody plants are very suitable to be used for ornamental purposes. Since shrubs are characterised, as Givnish [13] recalled, by a high number of active meristems, which are potential sites for stem regeneration, they are able to tolerate abiotic stresses more than trees. It is no coincidence, in fact, that shrubs are associated with degraded environments, where abiotic stresses are very frequent, for these reasons. They are plant species suitable for low-maintenance green infrastructures. Shrubs, perennial plants with numerous branches that branch out at or near the ground [1], are widespread in the different biomes of the Earth; their tolerance to numerous stresses allows them to ensure numerous ecosystem services [14]. These plant species are able to control temperatures, stabilize the soil, and ensure the water balance of the ecosystem, absorption, and carbon storage. The capacity to assure ecosystem services is crucial in the choice of ornamental plants for sustainable green infrastructures. From an ornamental point of view, shrubs are characterised by different features (high number of twigs, which influences their growth pattern; pulvinate shapes, which reduces their transpiration and improves the visual qualities of the green infrastructures; leaf characteristics; different blooming periods; and colours of flowers) that add interest and variety to the landscape [15].
2. Ornamental Shrubby Plants in the Urban Environment
Urbanisation significantly modifies the physical environment, biological components, and ecosystem processes of cities [16]. Woody plants, trees, and shrubs become essential components of urban green infrastructures for their numerous ecosystem services and, in particular, for the reduction of pollution [14]. Another aspect linked to urban environment quality is heat island reduction, determined by green areas and vegetation, particularly important in relation to increasing global warming. Plants, thanks to their shade [17] and transpiration, are able to improve urban conditions for the inhabitants but also for tourists who favour areas with green infrastructure to spend their time outdoors [18][19]. It is well-known that urban areas have temperatures 5–7 °C higher than rural areas. Mitigation of the high temperatures is obtained by the transpiration of plants, and the efficiency depends on water availability. Ornamental shrubs are able to maintain environmental quality and offer pleasant landscape effects, even if urbanisation, with its environmental changes, exerts negative effects on plants, which mainly affect the characteristics of the leaves [20]. At the same time, the variations that, due to the stressful effects of urbanisation, are observed in plants, such as leaf thickness, unit leaf area, specific leaf area, etc., can be used as indicators of the urban environment quality [20].
3. Mechanism of Tolerance and/or Resistance of Ornamental Shrubs to Abiotic Stress
Abiotic stresses are the major limiting growth factor in urban and peri-urban areas. The identification and use of tolerant ornamental species allow the reduction of management costs and preserve the aesthetical value of green areas. Urban environments can be subjected to more stressful conditions than rural areas [14].
Drought tolerance is most important for agricultural production, as most of the plants are obtained in Mediterranean, semi-arid, and tropical regions [21]. Soil water limitation during drought affects evaporation, evapotranspiration, and ultimately, precipitation [22]. Plants have developed various adaptive strategies to cope with drought stress [23]. Plants adapt to drought in several ways, such as drought escape, tolerance, and avoidance mechanisms [24]. Perennials especially rely on drought tolerance [25], which can be achieved through morphological adaptations of roots, stems, and leaves. Tolerant plants have a high-water potential with higher water uptake or physiological adaptations through the reduction of transpiration [26]. Plant adaptation varies among plants depending on species, genotype, phenological development, or organ type (leaves) [27]. Optimisation of carbon assimilation with minimisation of water loss, i.e., improvement of intrinsic water use efficiency, has been described as an adaptive trait for plants that are exposed to severe drought, like, for example, the Mediterranean woody plant species [28][29].
Woody species are particularly important in this context, due to their longevity and the possibility of studying long-term adaptation mechanisms. To understand the tolerance level, many physiological traits such as the measurement of leaf water potential before sunrise and at midday, photosynthetic rate, stomatal conductance, transpiration rate, and intercellular carbon dioxide concentration were analysed. Biochemical characteristics, such as ascorbic acid, glutathione, chlorophyll content, tocopherols, amino acids, carotenoids, and soluble sugar, have also been used to control the tolerance level of plants to drought stress [30]. It has been observed that species that can retain a greater quantity of water and therefore lose less through the stomata are more tolerant to drought [31]. As reported by Galmes et al. [32], shrubs have a better ability to regulate transpiration than herbaceous plants [30].
Many of the favourable characteristics for resisting drought are present in shrubs; it is not a coincidence that in semi-arid environments, most of the plants are sclerophyllous evergreen shrubs, or deciduous or seasonally dimorphic shrubs, which possess the main adaptive approaches of perennial species to drought stress [33]. The main role of shrubs in semi-arid ecosystems lies in the fact that these plants can grow under conditions of environmental stress where trees cannot survive [34][35]. Some perennial species, such as Euphorbia dendroides L., a Mediterranean shrub, keep their leaves during the winter and/or spring and drop them with the onset of the hot season.
The use of Mediterranean shrubs for revegetation in semi-arid areas has increased because of their ability to adapt to severe drought conditions, which is considered to be one of the most important factors influencing plant survival and species distribution [36]. In the case of Mediterranean evergreens, leaves undergo several drought events that can further hinder photosynthetic capacity [37][38]. One of their most distinctive characteristics is a higher water use efficiency (WUE) at the leaf level, due to the reduced stomatal conductance but higher carboxylation capacity of Rubisco compared to evergreens of other biomes [39]. Hence, stomatal and mesophyll diffusion constraints are the most important factors limiting photosynthesis in evergreens [40]. However, Mediterranean sclerophylls are able to sustain positive CO2 assimilation rates at relatively low leaf water potentials compared to Mediterranean deciduous species [39]. This increased drought tolerance has been partly attributed to the robustness of sclerophyll leaves [41], which tend to sustain shrinkage and collapse, thus preventing negative effects on photosynthesis and water transport [38][42]. Beyond the response mechanisms to drought stress, ornamental plants used in landscaping must ensure an aesthetic value that can be influenced by a reduction in the number of flowers, an excessive decrease in plant growth, and a worsening of foliage quality [30]. The analysis of the mechanisms adopted by different species to overcome drought stress and reduce water loss could allow the identification of the most tolerant species to be used in arid and semi-arid environments, thus increasing the sustainability of ornamental green infrastructures (Table 1).
Table 1. Effect of drought on ornamental plant quality and traits associated to tolerance.
| Target Organs |
Stress Effects | Tolerance or Adaptation Response |
References |
|---|---|---|---|
| Roots | Increase of root biomass | Increase the functional roots and architectures | [30] |
| Stem | Decrease the growth, elongation, diameter and biomass | Increase the lignification process (chi lo dice?) | [30][43][44] |
| Leaves | Reduction of size and leaf number | Increase the wax or thickness, and trichome number | [27][45] |
| Flowers | Reduction of flower production and longevity | Increase the flower longevity and turnover | [46] |
Salt stress is another important abiotic stress that ornamental plants and shrubs in landscaping can be exposed to. There are not numerous studies on the effects of saline stress [47]. Salinity can affect the growth of ornamental shrubs by reducing leaf growth and expansion due to osmotic effects or by toxicity due to the high concentration of Na+ and Cl− in saline water [48]. In ornamental plants, the aesthetic value can be compromised by salinity inducing leaf necrosis or abscission [49][50]. In many ornamental species, salinity usually induces dry shoot biomass and leaf surface. Morphological adaptations such as resinous buds, and waxy leaves and stems in tolerant species allow woody plants to cope with salinity stress. The salt exclusion mechanisms are represented by smooth twigs, sunken buds, and low surface area to volume ratios (as occurs, for example, in pine needles) [51][52].
Exposure to salt can affect plant metabolism through an osmotic effect, causing water deficit, or through a specific ion effect, causing excessive ion accumulation [53]. Under saline conditions, plants must activate various physiological and biochemical mechanisms to cope with saline stress, which include water relationships, photosynthesis rate, hormonal profiles, toxic ion distribution, antioxidant metabolism, and soil response [54]. In particular, the changes in leaf tissue cell walls and factors limiting photosynthesis under these conditions and their possible interactions with leaf tissue damage are not well understood [29]. Plants that have some degree of tolerance to salinity may show quality reductions when exposed to this stress, and this is an important factor in the selection of ornamental plants for use in gardens and landscaping [55].
The ionic composition of irrigation water can influence the response of shrubs and trees to salt stress. Chloride salts appear to be more harmful than SO42− salts, and Mg2+ associated with Cl− is more harmful than Na+ with Cl− [56]. Among many salinity tolerance mechanisms [57], the ability to limit the entry of saline ions through the roots and to limit the transport of Na+ and/or Cl− to the aerial parts, retaining these ions in the root and in the lower part of the stem, is one of the most important characteristics associated with salt tolerance [58]. Species that maintain acceptable growth rates under saline conditions have effective mechanisms for excluding Na+ and Cl− from roots or leaves, thus maintaining good aesthetics and are ideal for landscaping. The low reduction and absence of symptoms of salt damage in Eugenia myrtifolia L. was associated not only with the root storage of Na+ and Cl−, but also with their limited uptake with increasing salinity [59]. An important aspect of salt tolerance is related to a plant’s ability to compartmentalise toxic ions, such as Na+ and Cl−, in roots or stems [60][61].
The response of plants to salinity depends not only on the intensity of the salt treatment but also on the time of exposure to the salt treatment [62]. These aspects are of primary importance, especially in the Mediterranean area when saline water is used for irrigation of perennial species, such as woody plants, as the interaction between intensity and duration of exposure to salt will determine physiological and molecular changes. At nursery level, the selection of plants tolerant to salinity stress can be carried out by the evaluation of plants’ responses to salinity treatments (Table 2).
Table 2. Effect of salinity on ornamental plant quality and traits associated to tolerance.
| Target Organs |
Stress Effects | Tolerance or Adaptation Response |
Reference |
|---|---|---|---|
| Roots | Increase of roots biomass | Increase the water uptake and exclusion of some toxic ions such as Na+ or Cl− | [63] |
| Stem | Decrease the growth and biomass | Increase the extrusion or storage | [64] |
| Leaves | Reduction of size, necrosis, or abscission | Increase the storage of ions in vacuole | [49] |
| Flowers | Reduction of flowers production and longevity | Increase the flower longevity and turnover | [65] |
In urban areas, hypoxia is an abiotic stress that ornamental plants can be often exposed to in compacted soil. Compaction is determined by physical degradation, which reduces the volume of a given mass of soil and decreases porosity. This promotes the formation of urban flooding [66]. An excess of water is usually considered to be deleterious to plant health and growth, and total submergence rapidly kills most plant species. Hypoxia/anoxia conditions have negative effects on several biological processes such as plant respiration and water and nutrient absorption [67][68][69]. The most common symptoms in the aerial part of a plant under hypoxia/anoxia conditions include leaf curling (epinasty) and stem twisting, leaf chlorosis and wilting, marginal browning of the leaf and shedding/defoliation, as well as fruit drop. The physiological consequences of hypoxia are a decrease in stomatal conductance [70] and a reduction of water potential [71]. In woody plants, waterlogging tolerance responses are associated with hypertrophied lenticels, new adventitious roots, and aerenchyma development [72]. These morphological and anatomical changes depend on the intensity, duration, and timing of the flooding cycle [68]. The presence of hypertrophied lenticels is a common anatomical change observed in many woody species [73]. The development of hypertrophied lenticels is supposed to simplify the downward diffusion of O2 as well as the potential discharge of compounds produced in the roots as by-products of anaerobic metabolism [74].
Oxygen depletion is one of the most important events during flooding. The diminishing in gas diffusion to the root environment as a result of the presence of excessive water in the soil or deprived aeration in soilless cultivations, accompanied by reduction of available oxygen by aerobic processes (i.e., root and microbial respiration), will deprive the rhizosphere of available O2.
A flood-tolerant plant can overcome the adverse effects of flooding through numerous morphological modifications, such as hyponasty (upward bending of leaves), improved shoot extension, aerenchyma formation, the development of barriers against radial O2 loss (ROL) in roots, the development of adventitious roots, leaf anatomical changes, and the formation of a gas film on leaf surfaces [75][76]. The formation of adventitious roots improves the plant’s adaptation to flooding stress, effectively transports atmospheric O2 into the roots, and may support or replace the primary root system [75].
Urban environment pollution is also a source of stress in ornamental plants. Urban areas can be highly polluted by human activities. Pollution can be represented by heavy metals derived from heating systems, vehicular traffic, and industrial emissions [77]. Combustion of engines and tire emissions can represent a mobile pollutant source, while industries and heating systems represent fixed sources of pollution [14]. Around pollution sites, the concentration of heavy metals increases. Ornamental plants can have different degrees of pollution tolerance or ability to uptake and degrade them if they are organic pollutants. The use of suitable plant species can recover the visual appearance of polluted areas. The success of green area establishment depends on the tolerance of ornamental plants to the pollutant concentrations. Heavy metals are represented by different elements such as aluminium (Al), arsenic (As), cadmium (Cd), copper (Cu), chromium (Cr), lead (Pb), mercury (Hg), and zinc (Zn). The tolerance of ornamental plants to heavy metals is strictly connected with the ability of these species to exclude the toxic heavy metals from the uptake or the ability of plants to uptake and translocate heavy metals to organs that can be released, such as older leaves or even fruits [78].
The identification of tolerant ornamental plants at nursery level can be performed by exposing the interested species to increasing concentrations of heavy metals. The biochemical and physiological response of plants allows their classification to different levels of tolerance. The specific markers for the evaluation of tolerance to heavy metals can be the production and accumulation of some protection molecules such as phytochelatins. These proteins can protect the plants by removing metals from the active cell metabolism by chelation, and their biosynthesis is induced by heavy metals accumulation. The phytochelatin biosynthesis is mediated by phytochelatin synthase and starts from glutathione [79]. The activity of this enzyme is regulated by the heavy metals post-translation activation [80]. Heavy metals induce plant stress with the accumulation of free radicals and damage to the cell membrane. The most common radicals are represented by reactive oxygen species (ROS), reactive nitrogen species (RNS), or reactive sulfur species (RSS). The non-specific response can be represented by the increase of the detoxification enzymes such as those belonging to the ascorbate–glutathione cycle.
Free radicals are highly reactive and can damage the cell membrane and the phospholipid double layers. The damage of ROS on the cell membrane is specifically due to loss of compartment integrity and enzymes coming into contact with substrates generating products that can be responsible for several physiological disorders, compromising the visual appearance [81]. Membrane integrity and low lipid peroxidation are also good markers for the estimation of ornamental plant tolerance to heavy metal concentrations. At the nursery level, the selection of plants tolerant to heavy metals is carried out by exposing the plants to increasing doses and monitoring the lipid peroxidation, phytochelatin accumulation, and enzymatic response. The distribution of plants in the planning area must be done considering the concentration and distribution of pollution in the soil.
The shadows of buildings or tall plants in green areas can have negative effects on other plants. Therefore, the combination of different plant species such as herbaceous plants, shrubs, and woody ornamentals must be carefully considered. The visual appearance and aesthetical quality of the area depend on the health status of plants and their correct distribution. It is important to identify the correct exposure to ensure adequate light intensity. Plant distribution and combination must be carried out considering their shading tolerance. Buildings and trees can be responsible for shading and light limitations. Many ornamental plants can have a plasticity degree that allows the adaptation of plants to lower light intensities. At the nursery level, the ornamental plants can be prepared for low-light environments by progressive light reduction using black nets with a shading percentage from 50 to 90%. The intensity of shading depends on the shading in the urban area. The shade adaptation must be achieved by slow light intensity reduction [82]. Plants under shade contribute to the ornamental value through the increase of chlorophyll concentration. At the physiological level, leaves under progressive shading intensity reduce the light compensation point (Figure 1). This means that a lower amount of light is required to compensate the respiration process [83]. Ornamental plants that have good light plasticity can be used for green planning in the shaded areas inside urban and peri-urban environments. If plants are not tolerant to shade, under shading conditions, the respiration can be higher than photosynthesis with a negative sugar accumulation balance in a 24 h period. This negative balance, if prolonged, can lead to plant death.
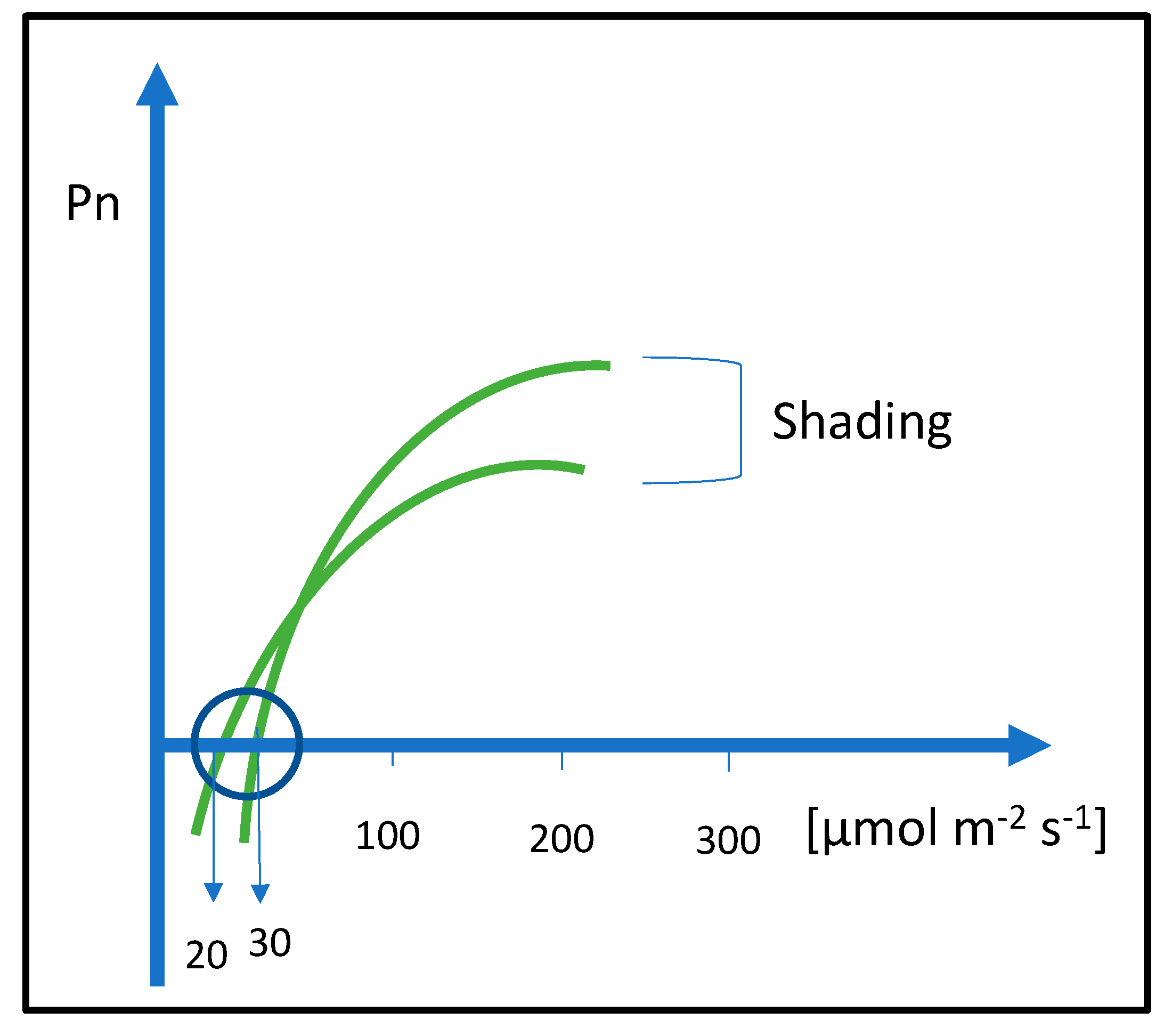
Figure 1. Schematic of light saturation curves and light compensation points lowered by shading treatments. Shading lowers the light compensation point from 30 to 20 µmol m−2 s−1. The lowering of light compensation point can require several days or weeks.
High and low temperatures can induce damages that compromise the ornamental value of plants. Temperature is an important environmental parameter that can induce speciation and affect plant distribution in diverse geographical areas. Plant growth and development are tightly correlated with temperature, and many species are synchronised with the environment for foliation and flowering. Each species has an optimal range of development; the minimum and maximum temperatures must be considered in the selection of plant species to use in certain regions or geographical areas. The temperature has a direct impact on primary and secondary metabolism. In an urban context, the reduction of growth is not a problem if there is any change of visual or external quality. In fact, slow growth can reduce the cost of management due to pruning. Unfortunately, the wrong ornamental plant selection exposed to low temperature can suffer cold stress or chilling injury during winter. On the contrary, plant species sensitive to high temperature, as well as for low temperature, can also show some physiological disorders such as leaf abscission or senescence.
Cold stress can be dramatically deleterious depending on the phenological stage of plants. Deciduous ornamental plants are strongly tolerant to low temperatures during winter when they are in the dormant stage. In spring, if new vegetation appears early, eventual low temperatures can induce chilling injury. Based on temperature data recorded in recent years, it is possible to distribute plants in areas considering their sensitivity to low temperatures. The combination of ornamental species from woody trees, shrubs, and herbaceous plants can protect each other.
References
- Götmark, F.; Götmark, E.; Jensen, A.M. Why be a shrub? A basic model and hypotheses for the adaptive values of a common growth form. Front. Plant Sci. 2016, 7, 1095.
- Read, P.E.; Bavougian, C.M. Woody ornamentals. In Horticulture: Plants for People and Places; Dixon, G., Aldous, D., Eds.; Springer: Dordrecht, The Netherland, 2014; Volume 2, pp. 619–644.
- Van Laere, K.; Hokanson, S.C.; Contreras, R.; Van Huylenbroeck, J. Woody ornamentals of the temperate zone. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 803–887.
- Lists of Names of Woody Plants and Perennials. Available online: https://www.naktuinbouw.com/lists-names (accessed on 15 January 2023).
- Paz, S.; Negev, M.; Clermont, A.; Green, M.S. Health aspects of climate change in cities with Mediterranean climate, and local adaptation plans. Int. J. Environ. Res. Public Health 2016, 13, 438.
- World Water Assessment Programme. The United Nations World Water Development Report 2014: Water and Energy; UNESCO: Paris, France, 2014; Available online: https://www.unwater.org/publications/un-world-water-development-report-2014 (accessed on 15 January 2023).
- Munné-Bosch, S.; Peñuelas, J. Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci. 2004, 166, 1105–1110.
- Cassaniti, C.; Romano, D.; Hop, M.E.C.M.; Flowers, T.J. Growing floricultural crops with brackish water. Environ. Exp. Bot. 2013, 92, 165–175.
- Filella, I.; Llusia, J.; Piñol, J.; Peñuelas, J. Leaf gas exchange and fluorescence of Phillyrea latifolia, Pistacia lentiscus and Quercus ilex saplings in severe drought and high temperature conditions. J. Exp. Bot. 1998, 39, 213–220.
- Medail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127.
- Heywood, V.; Skoula, M. The MEDUSA Network: Conservation and sustainable use of wild plants of the Mediterranean region. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 148–151.
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513.
- Givnish, T.J. Leaf and Canopy Adaptations in Tropical Forests. In Physiological Ecology of Plants of the Wet Tropics: Tasks for Vegetation Science; Medina, E., Mooney, H.A., Vázquez-Yánes, C., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 12, pp. 51–84.
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of ornamental plants to urban ecosystem services. Earth 2022, 3, 1258–1274.
- Romano, D.; Scariot, V. Woody ornamentals: A review of genetic resources in the Mediterranean area. Acta Hortic. 2021, 1331, 325–334.
- McDonald, A.G.; Bealey, W.J.; Fowler, D.; Dragosits, U.; Skiba, U.; Smith, R.I.; Nemitz, E. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos. Environ. 2007, 41, 8455–8467.
- Massetti, L.; Petralli, M.; Napoli, M.; Brandani, G.; Orlandini, S.; Pearlmutter, D. Effects of deciduous shade trees on surface temperature and pedestrian thermal stress during summer and autumn. Int. J. Biometeorol. 2019, 63, 467–479.
- Lopes, H.S.; Remoaldo, P.C.; Ribeiro, V.; Martin-Vide, J. Pathways for adapting tourism to climate change in an urban destination–Evidences based on thermal conditions for the Porto Metropolitan Area (Portugal). J. Environ. Manag. 2022, 315, 115161.
- Lopes, H.S.; Remoaldo, P.C.; Ribeiro, V.; Martín-Vide, J. A comprehensive methodology for assessing outdoor thermal comfort in touristic city of Porto (Portugal). Urban Clim. 2022, 45, 101264.
- Ilyas, M.; Liu, Y.Y.; Shah, S.; Ali, A.; Khan, A.H.; Zaman, F.; Zhang, Y.; Saud, S.; Adnan, M.; Wang, Y.J.; et al. Adaptation of functional traits and their plasticity of three ornamental trees growing in urban environment. Sci. Hortic. 2021, 286, 110248.
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303.
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of drought stress on crop production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 35–47.
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393.
- Levitt, J. Responses of Plants to Environmental Stresses. Volume II: Water, Radiation, Salt, and Other Stresses; Academic Press: New York, NY, USA, 1980; p. 607.
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334.
- Vinod, K.K. Stress in plantation crops: Adaptation and management. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherland, 2012; pp. 45–137.
- Chen, J.W.; Zhang, Q.; Li, X.S.; Cao, K.F. Independence of stem and leaf hydraulic traits in six Euphorbiaceae tree species with contrasting leaf phenology. Planta 2009, 230, 459–468.
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29.
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of Pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262.
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6.
- Riaz, A.; Younis, A.; Taj, A.R.; Karim, A.; Tariq, U.; Munir, S.; Riaz, S. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 2013, 45 (Suppl. S1), 123–131.
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93.
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392.
- Wilson, B.F. Shrub stems: Form and function. In Plant Stems Physiology and Functional Morphology; Gartner, B.L., Ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 91–102.
- Iqbal, A.; Fahad, S.; Iqbal, M.; Alamzeb, M.; Ahmad, A.; Anwar, S.; Khan, A.A.; Arif, M.; Saeed, M.; Song, M. Special adaptive features of plant species in response to drought. In Salt and Drought Stress Tolerance in Plants: Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 77–118.
- Vilagrosa, A.; Hernández, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological differences explain the co-existence of different regeneration strategies in Mediterranean ecosystems. New Phytol. 2014, 201, 1277–1288.
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41.
- Nadal, M.; Roig-Oliver, M.; Bota, J.; Flexas, J. Leaf age-dependent elastic adjustment and photosynthetic performance under drought stress in Arbutus unedo seedlings. Flora 2020, 271, 151662.
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23.
- Nadal, M.; Flexas, J. Variation in photosynthetic characteristics with growth form in a water-limited scenario: Implications for assimilation rates and water use efficiency in crops. Agric. Water Manag. 2019, 216, 457–472.
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin, Germany, 2012; pp. 37–61.
- Scoffoni, C.; Vuong, C.; Diep, S.; Cochard, H.; Sack, L. Leaf shrinkage with dehydration: Coordination with hydraulic vulnerability and drought tolerance. Plant Physiol. 2014, 164, 1772–1788.
- Jafari, S.; Hashemi Garmdareh, S.E.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133.
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309.
- Aspelmeier, S.; Leuschner, C. Genotypic variation in drought response of silver birch (Betula pendula Roth): Leaf and root morphology and carbon partitioning. Trees 2006, 20, 42–52.
- Cai, X.; Starman, T.; Niu, G.; Hall, C.; Lombardini, L. Response of selected garden roses to drought stress. HortScience 2012, 47, 1050–1055.
- Toscano, S.; Branca, F.; Romano, D.; Ferrante, A. An evaluation of different parameters to screen ornamental shrubs for salt spray tolerance. Biology 2020, 9, 250.
- USEPA. Manual: Guidelines for Water Reuse; USEPA, Rep. 625/R-92/004; USEPA: Washington, DC, USA, 1992. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2004-guidelines-water-reuse.pdf (accessed on 10 February 2023).
- Cassaniti, C.; Romano, D.; Flowers, T.J. The response of ornamental plants to saline irrigation water. In Irrigation: Water Management, Pollution and Alternative Strategies; Garcia-Garizabal, I., Abrahao, R., Eds.; IntechOpen Limited: London, UK, 2012; pp. 131–158.
- Percival, G.C. Identification of foliar salt tolerance of woody perennials using chlorophyll fluorescence. HortScience 2005, 40, 1892–1897.
- Appleton, B.; Huff, R.R.; French, S.C. Evaluating trees for saltwater spray tolerance for oceanfront sites. J. Arboric. 1999, 25, 205–210.
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of water stress on gas exchange, water relations and leaf structure in two ornamental shrubs in the Mediterranean area. Agronomy 2019, 9, 381.
- Azza Mazher, A.M.; Fatma El-Quesni, E.M.; Farahat, M.M. Responses of ornamental plants and woody trees to salinity. World J. Agric. Sci. 2007, 3, 386–395.
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18.
- Cameron, R.W.F.; Harrison-Murray, R.S.; Scott, M.A. The use of controlled water stress to manipulate growth of container-grown Rhododendron cv. Hoppy. J. Hortic. Sci. Biotechnol. 1999, 74, 161–169.
- Devitt, D.A.; Morris, R.L.; Fenstermaker, L.K. Foliar damage, spectral reflectance, and tissue ion concentrations of trees sprinkle irrigated with waters of similar salinity but different chemical composition. HortScience 2005, 40, 819–826.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681.
- Colmer, T.D.; Munns, R.; Flowers, T.J. Improving salt tolerance of wheat and barley: Future prospects. Aust. J. Exp. Agric. 2005, 45, 1425–1443.
- Boursier, P.; Läuchli, A. Growth responses and mineral nutrient relations of salt stressed sorghum. Crop Sci. 1990, 30, 1226–1233.
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The effect of sodium chloride on ornamental shrubs. Sci. Hortic. 2009, 122, 586–593.
- Ferguson, L.; Grattan, S.R. How salinity damages citrus: Osmotic effects and specific ion toxicities. HortTechnology 2005, 15, 95–99.
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74.
- Sánchez-Blanco, M.J.; Álvarez, S.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Root System Response to Drought and Salinity: Root Distribution and Water Transport. In Root Engineering: Soil Biology; Morte, A., Varma, A., Eds.; Springer: Berlin, Germany, 2014; Volume 40, pp. 325–352.
- Álvarez, S.; Gómez-Bellot, M.J.; Castillo, M.; Banon, S.; Sánchez-Blanco, M.J. Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environ. Exp. Bot. 2012, 78, 138–145.
- Fornes, F.; Belda, R.M.; Carrión, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hortic. 2007, 113, 52–59.
- Yang, J.L.; Zhang, G.L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61, 30–46.
- Jackson, M.B. Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci. 1997, 2, 22–28.
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490.
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312.
- Folzer, H.; Dat, J.F.; Capelli, N.; Rieffel, D.; Badot, P.M. Response to flooding of sessile oak: An integrative study. Tree Physiol. 2006, 26, 759–766.
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54.
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259.
- Yamamoto, F.; Sakata, T.; Terazawa, K. Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol. 1995, 15, 713–719.
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An overview of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27.
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286.
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73.
- Asgari Lajayer, B.; Khadem Moghadam, N.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484.
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780.
- Kneer, R.; Zenk, M.H. Phytochelatins protect plant enzymes from heavy metal poisoning. Phytochemistry 1992, 31, 2663–2667.
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832.
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486.
- Middleton, L. Shade-tolerant flowering plants: Adaptations and horticultural implications. Acta Hortic. 2001, 552, 95–102.
- Fonteno, W.C.; McWilliams, E.L. Light compensation points and acclimatization of four tropical foliage plants. J. Am. Soc. Hortic. Sci. 1978, 103, 52–56.
More
Information
Subjects:
Anatomy & Morphology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
01 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




