The native flora of different Mediterranean countries, often woody species, was widely recognized for its ornamental potential. The shrubs, in particular, are a typology of plants very widespread in the Mediterranean environment and constituent the ‘Macchia’, the typical vegetation of this ecosystem. These plant species could be used to improve the ornamental value of urban and peri-urban green areas. Since urban areas can suffer from low-quality soil and limited resources, the selection of plants must be carefully considered. The most commonly used plants should have adequate tolerance to abiotic stress.
- urban environment
- shrubs
- green areas
- landscape
- abiotic stress
1. Introduction
2. Ornamental Shrubby Plants in the Urban Environment
3. Mechanism of Tolerance and/or Resistance of Ornamental Shrubs to Abiotic Stress
| Target Organs |
Stress Effects | Tolerance or Adaptation Response |
References |
|---|---|---|---|
| Roots | Increase of root biomass | Increase the functional roots and architectures | [30] |
| Stem | Decrease the growth, elongation, diameter and biomass | Increase the lignification process (chi lo dice?) | [30][43][44] |
| Leaves | Reduction of size and leaf number | Increase the wax or thickness, and trichome number | [27][45] |
| Flowers | Reduction of flower production and longevity | Increase the flower longevity and turnover | [46] |
| Target Organs |
Stress Effects | Tolerance or Adaptation Response |
Reference |
|---|---|---|---|
| Roots | Increase of roots biomass | Increase the water uptake and exclusion of some toxic ions such as Na+ or Cl− | [63] |
| Stem | Decrease the growth and biomass | Increase the extrusion or storage | [64] |
| Leaves | Reduction of size, necrosis, or abscission | Increase the storage of ions in vacuole | [49] |
| Flowers | Reduction of flowers production and longevity | Increase the flower longevity and turnover | [65] |
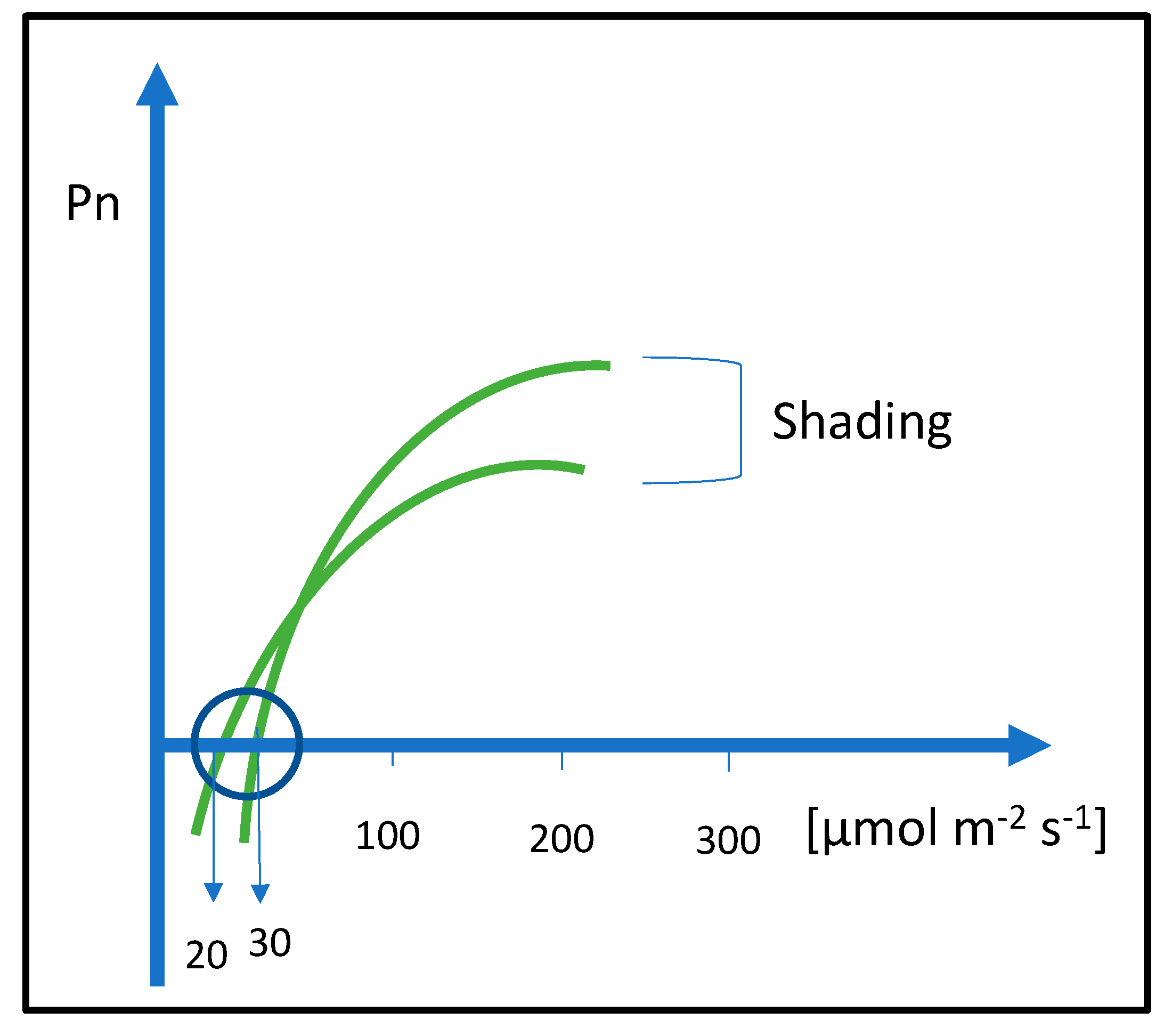
This entry is adapted from the peer-reviewed paper 10.3390/plants12102022
References
- Götmark, F.; Götmark, E.; Jensen, A.M. Why be a shrub? A basic model and hypotheses for the adaptive values of a common growth form. Front. Plant Sci. 2016, 7, 1095.
- Read, P.E.; Bavougian, C.M. Woody ornamentals. In Horticulture: Plants for People and Places; Dixon, G., Aldous, D., Eds.; Springer: Dordrecht, The Netherland, 2014; Volume 2, pp. 619–644.
- Van Laere, K.; Hokanson, S.C.; Contreras, R.; Van Huylenbroeck, J. Woody ornamentals of the temperate zone. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 803–887.
- Lists of Names of Woody Plants and Perennials. Available online: https://www.naktuinbouw.com/lists-names (accessed on 15 January 2023).
- Paz, S.; Negev, M.; Clermont, A.; Green, M.S. Health aspects of climate change in cities with Mediterranean climate, and local adaptation plans. Int. J. Environ. Res. Public Health 2016, 13, 438.
- World Water Assessment Programme. The United Nations World Water Development Report 2014: Water and Energy; UNESCO: Paris, France, 2014; Available online: https://www.unwater.org/publications/un-world-water-development-report-2014 (accessed on 15 January 2023).
- Munné-Bosch, S.; Peñuelas, J. Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci. 2004, 166, 1105–1110.
- Cassaniti, C.; Romano, D.; Hop, M.E.C.M.; Flowers, T.J. Growing floricultural crops with brackish water. Environ. Exp. Bot. 2013, 92, 165–175.
- Filella, I.; Llusia, J.; Piñol, J.; Peñuelas, J. Leaf gas exchange and fluorescence of Phillyrea latifolia, Pistacia lentiscus and Quercus ilex saplings in severe drought and high temperature conditions. J. Exp. Bot. 1998, 39, 213–220.
- Medail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127.
- Heywood, V.; Skoula, M. The MEDUSA Network: Conservation and sustainable use of wild plants of the Mediterranean region. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 148–151.
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean Basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513.
- Givnish, T.J. Leaf and Canopy Adaptations in Tropical Forests. In Physiological Ecology of Plants of the Wet Tropics: Tasks for Vegetation Science; Medina, E., Mooney, H.A., Vázquez-Yánes, C., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 12, pp. 51–84.
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of ornamental plants to urban ecosystem services. Earth 2022, 3, 1258–1274.
- Romano, D.; Scariot, V. Woody ornamentals: A review of genetic resources in the Mediterranean area. Acta Hortic. 2021, 1331, 325–334.
- McDonald, A.G.; Bealey, W.J.; Fowler, D.; Dragosits, U.; Skiba, U.; Smith, R.I.; Nemitz, E. Quantifying the effect of urban tree planting on concentrations and depositions of PM10 in two UK conurbations. Atmos. Environ. 2007, 41, 8455–8467.
- Massetti, L.; Petralli, M.; Napoli, M.; Brandani, G.; Orlandini, S.; Pearlmutter, D. Effects of deciduous shade trees on surface temperature and pedestrian thermal stress during summer and autumn. Int. J. Biometeorol. 2019, 63, 467–479.
- Lopes, H.S.; Remoaldo, P.C.; Ribeiro, V.; Martin-Vide, J. Pathways for adapting tourism to climate change in an urban destination–Evidences based on thermal conditions for the Porto Metropolitan Area (Portugal). J. Environ. Manag. 2022, 315, 115161.
- Lopes, H.S.; Remoaldo, P.C.; Ribeiro, V.; Martín-Vide, J. A comprehensive methodology for assessing outdoor thermal comfort in touristic city of Porto (Portugal). Urban Clim. 2022, 45, 101264.
- Ilyas, M.; Liu, Y.Y.; Shah, S.; Ali, A.; Khan, A.H.; Zaman, F.; Zhang, Y.; Saud, S.; Adnan, M.; Wang, Y.J.; et al. Adaptation of functional traits and their plasticity of three ornamental trees growing in urban environment. Sci. Hortic. 2021, 286, 110248.
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303.
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of drought stress on crop production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 35–47.
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393.
- Levitt, J. Responses of Plants to Environmental Stresses. Volume II: Water, Radiation, Salt, and Other Stresses; Academic Press: New York, NY, USA, 1980; p. 607.
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334.
- Vinod, K.K. Stress in plantation crops: Adaptation and management. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherland, 2012; pp. 45–137.
- Chen, J.W.; Zhang, Q.; Li, X.S.; Cao, K.F. Independence of stem and leaf hydraulic traits in six Euphorbiaceae tree species with contrasting leaf phenology. Planta 2009, 230, 459–468.
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29.
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of Pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262.
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6.
- Riaz, A.; Younis, A.; Taj, A.R.; Karim, A.; Tariq, U.; Munir, S.; Riaz, S. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 2013, 45 (Suppl. S1), 123–131.
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93.
- Toscano, S.; Ferrante, A.; Tribulato, A.; Romano, D. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 2018, 127, 380–392.
- Wilson, B.F. Shrub stems: Form and function. In Plant Stems Physiology and Functional Morphology; Gartner, B.L., Ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 91–102.
- Iqbal, A.; Fahad, S.; Iqbal, M.; Alamzeb, M.; Ahmad, A.; Anwar, S.; Khan, A.A.; Arif, M.; Saeed, M.; Song, M. Special adaptive features of plant species in response to drought. In Salt and Drought Stress Tolerance in Plants: Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 77–118.
- Vilagrosa, A.; Hernández, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological differences explain the co-existence of different regeneration strategies in Mediterranean ecosystems. New Phytol. 2014, 201, 1277–1288.
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41.
- Nadal, M.; Roig-Oliver, M.; Bota, J.; Flexas, J. Leaf age-dependent elastic adjustment and photosynthetic performance under drought stress in Arbutus unedo seedlings. Flora 2020, 271, 151662.
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23.
- Nadal, M.; Flexas, J. Variation in photosynthetic characteristics with growth form in a water-limited scenario: Implications for assimilation rates and water use efficiency in crops. Agric. Water Manag. 2019, 216, 457–472.
- De Micco, V.; Aronne, G. Morpho-anatomical traits for plant adaptation to drought. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin, Germany, 2012; pp. 37–61.
- Scoffoni, C.; Vuong, C.; Diep, S.; Cochard, H.; Sack, L. Leaf shrinkage with dehydration: Coordination with hydraulic vulnerability and drought tolerance. Plant Physiol. 2014, 164, 1772–1788.
- Jafari, S.; Hashemi Garmdareh, S.E.; Azadegan, B. Effects of drought stress on morphological, physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 253, 128–133.
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 2018, 178, 1296–1309.
- Aspelmeier, S.; Leuschner, C. Genotypic variation in drought response of silver birch (Betula pendula Roth): Leaf and root morphology and carbon partitioning. Trees 2006, 20, 42–52.
- Cai, X.; Starman, T.; Niu, G.; Hall, C.; Lombardini, L. Response of selected garden roses to drought stress. HortScience 2012, 47, 1050–1055.
- Toscano, S.; Branca, F.; Romano, D.; Ferrante, A. An evaluation of different parameters to screen ornamental shrubs for salt spray tolerance. Biology 2020, 9, 250.
- USEPA. Manual: Guidelines for Water Reuse; USEPA, Rep. 625/R-92/004; USEPA: Washington, DC, USA, 1992. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/2004-guidelines-water-reuse.pdf (accessed on 10 February 2023).
- Cassaniti, C.; Romano, D.; Flowers, T.J. The response of ornamental plants to saline irrigation water. In Irrigation: Water Management, Pollution and Alternative Strategies; Garcia-Garizabal, I., Abrahao, R., Eds.; IntechOpen Limited: London, UK, 2012; pp. 131–158.
- Percival, G.C. Identification of foliar salt tolerance of woody perennials using chlorophyll fluorescence. HortScience 2005, 40, 1892–1897.
- Appleton, B.; Huff, R.R.; French, S.C. Evaluating trees for saltwater spray tolerance for oceanfront sites. J. Arboric. 1999, 25, 205–210.
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of water stress on gas exchange, water relations and leaf structure in two ornamental shrubs in the Mediterranean area. Agronomy 2019, 9, 381.
- Azza Mazher, A.M.; Fatma El-Quesni, E.M.; Farahat, M.M. Responses of ornamental plants and woody trees to salinity. World J. Agric. Sci. 2007, 3, 386–395.
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18.
- Cameron, R.W.F.; Harrison-Murray, R.S.; Scott, M.A. The use of controlled water stress to manipulate growth of container-grown Rhododendron cv. Hoppy. J. Hortic. Sci. Biotechnol. 1999, 74, 161–169.
- Devitt, D.A.; Morris, R.L.; Fenstermaker, L.K. Foliar damage, spectral reflectance, and tissue ion concentrations of trees sprinkle irrigated with waters of similar salinity but different chemical composition. HortScience 2005, 40, 819–826.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681.
- Colmer, T.D.; Munns, R.; Flowers, T.J. Improving salt tolerance of wheat and barley: Future prospects. Aust. J. Exp. Agric. 2005, 45, 1425–1443.
- Boursier, P.; Läuchli, A. Growth responses and mineral nutrient relations of salt stressed sorghum. Crop Sci. 1990, 30, 1226–1233.
- Cassaniti, C.; Leonardi, C.; Flowers, T.J. The effect of sodium chloride on ornamental shrubs. Sci. Hortic. 2009, 122, 586–593.
- Ferguson, L.; Grattan, S.R. How salinity damages citrus: Osmotic effects and specific ion toxicities. HortTechnology 2005, 15, 95–99.
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74.
- Sánchez-Blanco, M.J.; Álvarez, S.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Root System Response to Drought and Salinity: Root Distribution and Water Transport. In Root Engineering: Soil Biology; Morte, A., Varma, A., Eds.; Springer: Berlin, Germany, 2014; Volume 40, pp. 325–352.
- Álvarez, S.; Gómez-Bellot, M.J.; Castillo, M.; Banon, S.; Sánchez-Blanco, M.J. Osmotic and saline effect on growth, water relations, and ion uptake and translocation in Phlomis purpurea plants. Environ. Exp. Bot. 2012, 78, 138–145.
- Fornes, F.; Belda, R.M.; Carrión, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hortic. 2007, 113, 52–59.
- Yang, J.L.; Zhang, G.L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61, 30–46.
- Jackson, M.B. Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci. 1997, 2, 22–28.
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490.
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312.
- Folzer, H.; Dat, J.F.; Capelli, N.; Rieffel, D.; Badot, P.M. Response to flooding of sessile oak: An integrative study. Tree Physiol. 2006, 26, 759–766.
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54.
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259.
- Yamamoto, F.; Sakata, T.; Terazawa, K. Physiological, morphological and anatomical responses of Fraxinus mandshurica seedlings to flooding. Tree Physiol. 1995, 15, 713–719.
- Parent, C.; Capelli, N.; Berger, A.; Crèvecoeur, M.; Dat, J.F. An overview of plant responses to soil waterlogging. Plant Stress 2008, 2, 20–27.
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286.
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73.
- Asgari Lajayer, B.; Khadem Moghadam, N.; Maghsoodi, M.R.; Ghorbanpour, M.; Kariman, K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: Mechanisms and efficiency improvement strategies. Environ. Sci. Pollut. Res. 2019, 26, 8468–8484.
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780.
- Kneer, R.; Zenk, M.H. Phytochelatins protect plant enzymes from heavy metal poisoning. Phytochemistry 1992, 31, 2663–2667.
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832.
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486.
- Middleton, L. Shade-tolerant flowering plants: Adaptations and horticultural implications. Acta Hortic. 2001, 552, 95–102.
- Fonteno, W.C.; McWilliams, E.L. Light compensation points and acclimatization of four tropical foliage plants. J. Am. Soc. Hortic. Sci. 1978, 103, 52–56.
