
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hina Arif- Arif-Tiwari | -- | 2056 | 2023-05-30 17:59:07 | | | |
| 2 | Peter Tang | + 1 word(s) | 2057 | 2023-05-31 03:03:17 | | |
Video Upload Options
Ultrasound and computed tomography (CT) have been the mainstay of renal mass screening and diagnosis but advances in magnetic resonance (MR) technology have made this the optimal choice when diagnosing and staging renal tumors.
1. Introduction
2. Imaging Modalities
2.1. Ultrasound
2.2. Computed Tomography
2.3. Magnetic Resonance
3. Magnetic Resonance Imaging Approach
3.1. Magnetic Resonance Imaging Protocol
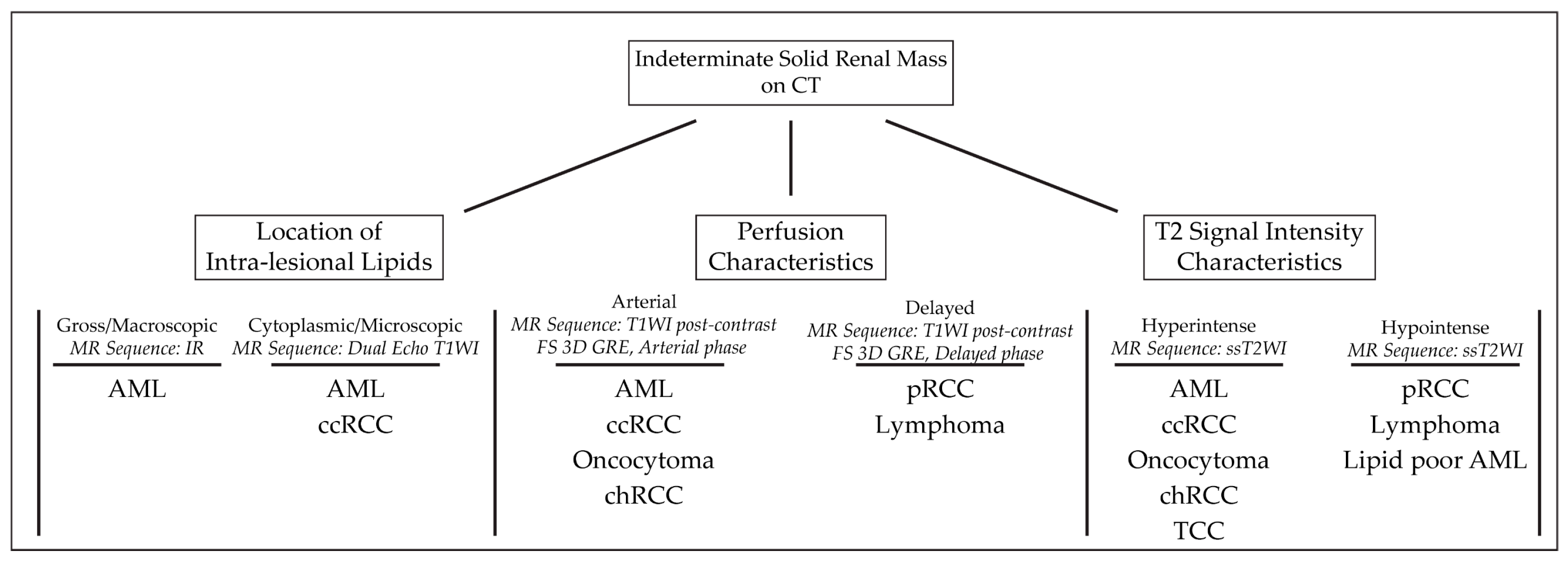
|
Type of Renal Masses |
|
|---|---|
|
Benign Masses |
|
|
Angiomyolipoma |
High T2-intensity signal due to fat content. Low T2 on fat-suppressed images. Microscopic, intracytoplasmic fat made apparent with in- and out-of-phase GRE |
|
Lipid-poor Angiomyolipoma |
T2-hypointense Macroscopic fat and/or absence of fat High arterial enhancement with subsequent washout |
|
Oncocytoma |
T2-iso-to-hyperintense relative to normal parenchyma Central/eccentric T2-hyperintense scar Delayed enhancement of a central scar Segmental enhancement inversion pattern |
|
Renal Cell Carcinomas |
|
|
Clear Cell RCC |
Heterogenous, high T2-intensity Avid enhancement in corticomedullary and nephrogenic phases Microscopic fat as see on dual echo T1W in- and out-of-phase Invasion into surrounding vessels (esp. renal vein or IVC) Presence of necrosis or intralesional calcification |
|
Type 1 Papillary RCC |
T2-hypointense Uniform progressive delayed enhancement Well-circumscribed, homogenous, peripherally-located |
|
Type 2 Papillary RCC |
Heterogenous T2 signal intensity Heterogenous enhancement Larger with more indistinct margin vs versus Type 1 pRCC |
|
Chromophobe RCC |
Low to intermediate T2-intensity Intermediate, delayed enhancement Central, stellate scar with “spoke-wheel” enhancement pattern Peripheral, homogenous, well-circumscribed Mimics oncocytoma on imaging |
|
Rare Renal Masses |
|
|
Renal Lymphoma |
Low to intermediate T1 and T2 signal intensity Mild, delayed, homogenous enhancement Multiple 1–3 cm solitary masses |
|
Metastasis |
Varied presentation, usually identical to primary tumor Multiple, atypical renal masses History of advanced, non-renal malignancy |
|
Transitional Cell Carcinoma |
Intermediate T1 and T2 signal intensity Delayed, heterogenous enhancement Filling defects and soft masses when urine is present as contrast medium |
References
- Laguna, B.; Westphalen, A.C.; Guimaraes, C.T.; Whang, Z.; Simko, J.; Zagoria, R. Uncommon malignant renal tumors and atypical presentation of common ones: A guide for radiologists. Abdom. Radiol. 2019, 44, 1430–1452.
- Turner, R.M., 2nd; Morgan, T.M.; Jacobs, B.L. Epidemiology of the Small Renal Mass and the Treatment Disconnect Phenomenon. Urol. Clin. N. Am. 2017, 44, 147–154.
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising incidence of small renal masses: A need to reassess treatment effect. J. Natl. Cancer Inst. 2006, 98, 1331–1334.
- Patel, H.D.; Gupta, M.; Joice, G.A.; Srivastava, A.; Alam, R.; Allaf, M.E.; Pierorazio, P.M. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur. Urol. Oncol. 2019, 2, 343–348.
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134.
- Hudes, G.; Carducci, M.; Tomczak, P.; Dutcher, J.; Figlin, R.; Kapoor, A.; Staroslawska, E.; Sosman, J.; McDermott, D.; Bodrogi, I.; et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281.
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124.
- Ball, M.W. Surgical management of metastatic renal cell carcinoma. Discov. Med. 2017, 23, 379–387.
- Cornelis, F.; Grenier, N. Multiparametric Magnetic Resonance Imaging of Solid Renal Tumors: A Practical Algorithm. Semin. Ultrasound CT MR 2017, 38, 47–58.
- Kang, S.K.; Huang, W.C.; Pandharipande, P.V.; Chandarana, H. Solid renal masses: What the numbers tell us. AJR Am. J. Roentgenol. 2014, 202, 1196–1206.
- Uzosike, A.C.; Patel, H.D.; Alam, R.; Schwen, Z.R.; Gupta, M.; Gorin, M.A.; Johnson, M.H.; Gausepohl, H.; Riffon, M.F.; Trock, B.J.; et al. Growth Kinetics of Small Renal Masses on Active Surveillance: Variability and Results from the DISSRM Registry. J. Urol. 2018, 199, 641–648.
- Li, G.; Cuilleron, M.; Gentil-Perret, A.; Tostain, J. Characteristics of image-detected solid renal masses: Implication for optimal treatment. Int. J. Urol. 2004, 11, 63–67.
- Silverman, S.G.; Pedrosa, I.; Ellis, J.H.; Hindman, N.M.; Schieda, N.; Smith, A.D.; Remer, E.M.; Shinagare, A.B.; Curci, N.E.; Raman, S.S.; et al. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology 2019, 292, 475–488.
- Chenam, A.; Lau, C. Management of Small Renal Masses. Cancer Treat Res. 2018, 175, 105–126.
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926.
- Heilbrun, M.E.; Remer, E.M.; Casalino, D.D.; Beland, M.D.; Bishoff, J.T.; Blaufox, M.D.; Coursey, C.A.; Goldfarb, S.; Harvin, H.J.; Nikolaidis, P.; et al. ACR Appropriateness Criteria indeterminate renal mass. J. Am. Coll. Radiol. 2015, 12, 333–341.
- van Oostenbrugge, T.J.; Futterer, J.J.; Mulders, P.F.A. Diagnostic Imaging for Solid Renal Tumors: A Pictorial Review. Kidney Cancer 2018, 2, 79–93.
- Jamis-Dow, C.A.; Choyke, P.L.; Jennings, S.B.; Linehan, W.M.; Thakore, K.N.; Walther, M.M. Small (< or = 3-cm) renal masses: Detection with CT versus US and pathologic correlation. Radiology 1996, 198, 785–788.
- Jinzaki, M.; Ohkuma, K.; Tanimoto, A.; Mukai, M.; Hiramatsu, K.; Murai, M.; Hata, J. Small solid renal lesions: Usefulness of power Doppler US. Radiology 1998, 209, 543–550.
- Abou Elkassem, A.M.; Lo, S.S.; Gunn, A.J.; Shuch, B.M.; Dewitt-Foy, M.E.; Abouassaly, R.; Vaidya, S.S.; Clark, J.I.; Louie, A.V.; Siva, S.; et al. Role of Imaging in Renal Cell Carcinoma: A Multidisciplinary Perspective. Radiographics 2021, 41, 1387–1407.
- Yuh, B.I.; Cohan, R.H. Different phases of renal enhancement: Role in detecting and characterizing renal masses during helical CT. AJR Am. J. Roentgenol. 1999, 173, 747–755.
- Krishna, S.; Murray, C.A.; McInnes, M.D.; Chatelain, R.; Siddaiah, M.; Al-Dandan, O.; Narayanasamy, S.; Schieda, N. CT imaging of solid renal masses: Pitfalls and solutions. Clin. Radiol. 2017, 72, 708–721.
- Mileto, A.; Nelson, R.C.; Paulson, E.K.; Marin, D. Dual-Energy MDCT for Imaging the Renal Mass. AJR Am. J. Roentgenol. 2015, 204, W640–W647.
- Canvasser, N.E.; Kay, F.U.; Xi, Y.; Pinho, D.F.; Costa, D.; de Leon, A.D.; Khatri, G.; Leyendecker, J.R.; Yokoo, T.; Lay, A.; et al. Diagnostic Accuracy of Multiparametric Magnetic Resonance Imaging to Identify Clear Cell Renal Cell Carcinoma in cT1a Renal Masses. J. Urol. 2017, 198, 780–786.
- Weinreb, J.C.; Rodby, R.A.; Yee, J.; Wang, C.L.; Fine, D.; McDonald, R.J.; Perazella, M.A.; Dillman, J.R.; Davenport, M.S. Use of Intravenous Gadolinium-based Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2021, 298, 28–35.
- Kay, F.U.; Pedrosa, I. Imaging of Solid Renal Masses. Urol. Clin. N. Am. 2018, 45, 311–330.
- Schieda, N.; Lim, R.S.; McInnes, M.D.F.; Thomassin, I.; Renard-Penna, R.; Tavolaro, S.; Cornelis, F.H. Characterization of small (<4 cm) solid renal masses by computed tomography and magnetic resonance imaging: Current evidence and further development. Diagn. Interv. Imaging 2018, 99, 443–455.




