
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cosima Chiara Hoch | -- | 2842 | 2023-05-29 23:51:34 | | | |
| 2 | Jessie Wu | + 50 word(s) | 2892 | 2023-05-30 05:51:45 | | |
Video Upload Options
Salivary adenoid cystic carcinoma (SACC) is considered the second most frequent malignant salivary gland neoplasm and exhibits a controversial and poorly understood biological behavior, characterized by slow and indolent growth. SACC typically arises from the submandibular gland and minor salivary glands, while its occurrence in the parotid gland is relatively rare. SACC has an incidence rate of approximately 4.5 cases per million individuals and constitutes 10% of all salivary gland tumors (SGTs). This type of cancer exhibits three distinct histological growth patterns, namely cribriform, tubular, and solid patterns. Among these patterns, the solid pattern represents the most aggressive form of SACC with an increased risk of metastasis, resulting in shorter disease-specific survival.
1. The Role of Epithelial–Mesenchymal Transition through the Activation of Transcription Factors in Cancer Invasion and Metastasis in Salivary Adenoid Cystic Carcinoma
1.1. TWIST Expression in Salivary Adenoid Cystic Carcinoma and Its Significance in Pathogenesis and Prognosis
1.2. SNAIL and SLUG: Key Transcription Factors Driving Epithelial–Mesenchymal Transition and Metastasis in Salivary Adenoid Cystic Carcinoma
1.3. ZEB1/ZEB2: A Key Regulator of Epithelial–Mesenchymal Transition in Salivary Adenoid Cystic Carcinoma
2. Expression of Major Epithelial–Mesenchymal Transition Markers in Salivary Adenoid Cystic Carcinoma
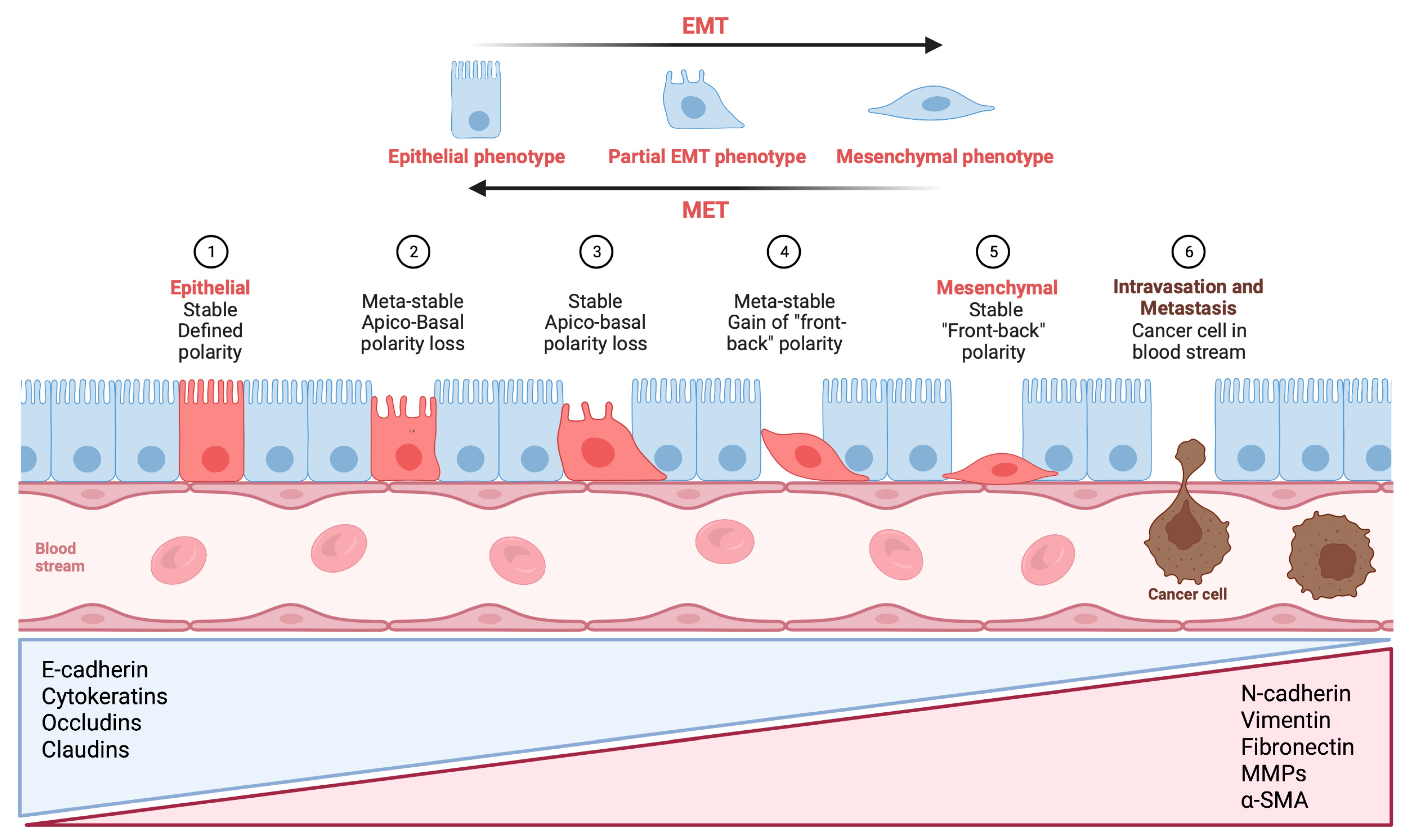
2.1. Expression of CK in Salivary Adenoid Cystic Carcinoma
2.2. The Role of E-Cadherin in Salivary Adenoid Cystic Carcinoma: Implications for Tumor Invasion, Metastasis, and Epithelial–Mesenchymal Transition
2.3. Vimentin: A Key Intermediate Filament in Mesenchymal Cells and Its Role in Salivary Adenoid Cystic Carcinoma Metastasis
2.4. The Complex Role of Fibronectin in Salivary Adenoid Cystic Carcinoma: Promoting Perineural Invasion, Inhibiting Invasion, and Potential Prognostic Marker
2.5. N-Cadherin Expression in Salivary Adenoid Cystic Carcinoma: Association with Perineural Invasion and Epithelial–Mesenchymal Transition
References
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494.
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890.
- Wang, S.M.; Coljee, V.W.; Pignolo, R.J.; Rotenberg, M.O.; Cristofalo, V.J.; Sierra, F. Cloning of the human twist gene: Its expression is retained in adult mesodermally-derived tissues. Gene 1997, 187, 83–92.
- Belulescu, I.C.; Mărgăritescu, C.; Dumitrescu, C.I.; Munteanu, M.C.; Dăguci, L.; Mărgăritescu, O.C.; Matei, M. The immunophenotype of epithelial to mesenchymal transition inducing transcription factors in salivary gland adenoid cystic carcinomas. Rom. J. Morphol. Embryol. 2020, 61, 769–782.
- Zhou, C.; Liu, J.; Tang, Y.; Zhu, G.; Zheng, M.; Jiang, J.; Yang, J.; Liang, X. Coexpression of hypoxia-inducible factor-2α, TWIST2, and SIP1 may correlate with invasion and metastasis of salivary adenoid cystic carcinoma. J. Oral Pathol. Med. 2012, 41, 424–431.
- Shen, M.; Wen, Y.; Hua, C.; Xiao, J. The expression of Twist in salivary adenoid cystic carcinoma and its clinicopathological significance. Chin. -Ger. J. Clin. Oncol. 2010, 9, 187–192.
- Pardis, S.; Zare, R.; Jaafari-Ashkavandi, Z.; Ashraf, M.J.; Khademi, B. Twist expression in pleomorphic adenoma, adenoid cystic carcinoma and mucoepidermoid carcinoma of salivary glands. Turk. J. Pathol. 2016, 32, 15–21.
- Kerche, L.E.; de Sousa, E.A.; Squarize, C.H.; Oliveira, K.K.; Marchi, F.A.; Bettim, B.B.; Kowalski, L.P.; Soares, F.A.; Lourenço, S.V.; Coutinho-Camillo, C.M. EMT in salivary gland tumors: The expression of microRNAs miR-155 and miR-200c is associated with clinical-pathological parameters. Mol. Biol. Rep. 2022, 49, 2157–2167.
- Silva, B.S.D.F.; Silva, F.P.Y.; Pontes, H.A.R.; Junior, D.D.S.P. E-cadherin downregulation and Twist overexpression since early stages of oral carcinogenesis. J. Oral Pathol. Med. 2014, 43, 125–131.
- Yuen, H.-F.; Chua, C.-W.; Chan, Y.-P.; Wong, Y.-C.; Wang, X.; Chan, K.-W. Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology 2007, 50, 648–658.
- Zhang, M.; Zheng, M.; Dai, L.; Zhang, W.; Fan, H.; Yu, X.; Pang, X.; Liao, P.; Chen, B.; Wang, S.; et al. CXCL12/CXCR4 facilitates perineural invasion via induction of the Twist/S100A4 axis in salivary adenoid cystic carcinoma. J. Cell. Mol. Med. 2021, 25, 7901–7912.
- Yokoyama, K.; Kamata, N.; Hayashi, E.; Hoteiya, T.; Ueda, N.; Fujimoto, R.; Nagayama, M. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001, 37, 65–71.
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; De Herreros, A.G. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89.
- Jiang, J.; Tang, Y.; Zhu, G.; Zheng, M.; Yang, J.; Liang, X. Correlation between transcription factor Snail1 expression and prognosis in adenoid cystic carcinoma of salivary gland. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 764–769.
- Zhao, D.; Yang, K.; Tang, X.-F.; Lin, N.-N.; Liu, J.-Y. Expression of integrin-linked kinase in adenoid cystic carcinoma of salivary glands correlates with epithelial–mesenchymal transition markers and tumor progression. Med. Oncol. 2013, 30, 619.
- Chen, W.; Ren, X.; Wu, J.; Gao, X.; Cen, X.; Wang, S.; Sheng, S.; Chen, Q.; Tang, Y.; Liang, X. HSP 27 associates with epithelial–mesenchymal transition, stemness and radioresistance of salivary adenoid cystic carcinoma. J. Cell. Mol. Med. 2018, 22, 2283–2298.
- Du, Y.; Lv, D.; Cui, B.; Li, X.; Chen, H.; Kang, Y.; Chen, Q.; Feng, Y.; Zhang, P.; Chen, J.; et al. Protein kinase D1 induced epithelial–mesenchymal transition and invasion in salivary adenoid cystic carcinoma via E-cadherin/Snail regulation. Oral Dis. 2022, 28, 1539–1554.
- Tang, Y.; Liang, X.; Zhu, G.; Zheng, M.; Yang, J.; Chen, Y. Expression and importance of zinc-finger transcription factor Slug in adenoid cystic carcinoma of salivary gland. J. Oral Pathol. Med. 2010, 39, 775–780.
- He, Q.; Zhou, X.; Li, S.; Jin, Y.; Chen, Z.; Chen, D.; Cai, Y.; Liu, Z.; Zhao, T.; Wang, A. MicroRNA-181a suppresses salivary adenoid cystic carcinoma metastasis by targeting MAPK–Snai2 pathway. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5258–5266.
- Wu, B.; Wei, J.; Hu, Z.; Shan, C.; Wang, L.; Zhang, C.; Yang, X.; Yang, X.; Lei, D. Slug silencing inhibited perineural invasion through regulation of EMMPRIN expression in human salivary adenoid cystic carcinoma. Tumor Biol. 2016, 37, 2161–2169.
- Yang, L.; Wang, T.; Zhang, J.; Wang, X. BTBD7 silencing inhibited epithelial- mesenchymal transition (EMT) via regulating Slug expression in human salivary adenoid cystic carcinoma. Cancer Biomark. 2017, 20, 461–468.
- Yang, L.; Wang, T.; Zhang, J.; Liu, Z.; Wang, X. Expression of BTBD7 in primary salivary adenoid cystic carcinoma and correlation with Slug and prognosis. Cancer Biomark. 2016, 17, 179–185.
- Liu, S.; Ye, D.; Xu, D.; Liao, Y.; Zhang, L.; Liu, L.; Yu, W.; Wang, Y.; He, Y.; Hu, J.; et al. Autocrine epiregulin activates EGFR pathway for lung metastasis via EMT in salivary adenoid cystic carcinoma. Oncotarget 2016, 7, 25251–25263.
- Wang, Y.; Hu, J.; Wang, Y.; Ye, W.; Zhang, X.; Ju, H.; Xu, D.; Liu, L.; Ye, D.; Zhang, L.; et al. EGFR activation induced Snail-dependent EMT and myc-dependent PD-L1 in human salivary adenoid cystic carcinoma cells. Cell Cycle 2018, 17, 1457–1470.
- Jiang, Y.; Ge, X.-Y.; Liu, S.-M.; Zheng, L.; Huang, M.-W.; Shi, Y.; Fu, J.; Zhang, J.-G.; Li, S.-L. Nimotuzumab suppresses epithelial–mesenchymal transition and enhances apoptosis in low-dose UV-C treated salivary adenoid cystic carcinoma cell lines in vitro. Anti-Cancer Drugs 2014, 25, 1052–1060.
- Yi, C.; Li, B.-B.; Zhou, C.-X. Bmi-1 expression predicts prognosis in salivary adenoid cystic carcinoma and correlates with epithelial-mesenchymal transition–related factors. Ann. Diagn. Pathol. 2016, 22, 38–44.
- Xu, X.M.; Liu, W.; Cao, Z.-H.; Liu, M.-X. Effects of ZEB1 on regulating osteosarcoma cells via NF-κB/iNOS. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1184–1190.
- Gu, K.; Ming-Ming, L.; Jing, S.; Fang, L.; Jing-Yan, C.; Shi, J.; Yan, Y. Interleukin-17-induced EMT promotes lung cancer cell migration and invasion via NF-κB/ZEB1 signal pathway. Am. J. Cancer Res. 2015, 5, 1169–1179.
- Yao, X.; Wang, Y.; Duan, Y.; Zhang, Q.; Li, P.; Jin, R.; Tao, Y.; Zhang, W.; Wang, X.; Jing, C.; et al. IGFBP2 promotes salivary adenoid cystic carcinoma metastasis by activating the NF-κB/ZEB1 signaling pathway. Cancer Lett. 2018, 432, 38–46.
- Peng, J.; Wang, H.-C.; Liu, Y.; Jiang, J.-H.; Lv, W.-Q.; Yang, Y.; Li, C.-Y.; Qiu, X.-Y. Involvement of non-B cell-derived immunoglobulin G in the metastasis and prognosis of salivary adenoid cystic carcinoma. Oncol. Lett. 2017, 14, 4491–4498.
- Slack, J.M.; Tosh, D. Transdifferentiation and metaplasia—switching cell types. Curr. Opin. Genet. Dev. 2001, 11, 581–586.
- Matsumiya-Matsumoto, Y.; Morita, Y.; Uzawa, N. Pleomorphic Adenoma of the Salivary Glands and Epithelial–Mesenchymal Transition. J. Clin. Med. 2022, 11, 4210.
- Kuburich, N.A.; Hollander, P.D.; Pietz, J.T.; Mani, S.A. Vimentin and cytokeratin: Good alone, bad together. Semin. Cancer Biol. 2022, 86 Pt 3, 816–826.
- Belulescu, I.C.; Mărgăritescu, C.; Dumitrescu, C.I.; Munteanu, M.C.; Mărgăritescu, O.C. Immunophenotypical alterations with impact on the epithelial–mesenchymal transition (EMT) process in salivary gland adenoid cystic carcinomas. Rom. J. Morphol. Embryol. 2020, 61, 175–187.
- Meer, S.; Altini, M. CK7+/CK20? immunoexpression profile is typical of salivary gland neoplasia. Histopathology 2007, 51, 26–32.
- Lee, J.-H.; Lee, J.H.; Kim, A.; Kim, I.; Chae, Y.-S. Unique expression of MUC3, MUC5AC and cytokeratins in salivary gland carcinomas. Pathol. Int. 2005, 55, 386–390.
- Ben Salha, I.; Bhide, S.; Mourtzoukou, D.; Fisher, C.; Thway, K. Solid Variant of Adenoid Cystic Carcinoma: Difficulties in Diagnostic Recognition. Int. J. Surg. Pathol. 2016, 24, 419–424.
- Gao, X.-L.; Wu, J.-S.; Cao, M.-X.; Gao, S.-Y.; Cen, X.; Jiang, Y.-P.; Wang, S.-S.; Tang, Y.-J.; Chen, Q.-M.; Liang, X.-H.; et al. Cytokeratin-14 contributes to collective invasion of salivary adenoid cystic carcinoma. PLoS ONE 2017, 12, e0171341.
- Ge, M.-H.; Ling, Z.-Q.; Tan, Z.; Chen, C.; Xu, J.-J.; Yu, J.-L. Expression and significance of E-cadherin in adenoid cystic carcinoma of salivary glands. Zhonghua Yi Xue Za Zhi 2012, 92, 106–109.
- Lai, F.-Y.; Zhang, Q.; Wu, Q.-L.; Qing, J.; Cao, Y. Expression and significance of E-cadherin in adenoid cystic carcinoma of the salivary glands. Ai Zheng 2007, 26, 1025–1028.
- Maruya, S.-I.; Kurotaki, H.; Wada, R.; Saku, T.; Shinkawa, H.; Yagihashi, S. Promoter methylation and protein expression of the E-cadherin gene in the clinicopathologic assessment of adenoid cystic carcinoma. Mod. Pathol. 2004, 17, 637–645.
- Prabhu, S.; Kaveri, H.; Rekha, K. Benign; malignant salivary gland tumors: Comparison of immunohistochemical expression of e-cadherin. Oral Oncol. 2009, 45, 594–599.
- Zhang, M.; Li, Z.; Wang, H.; Wang, S.; Yu, X.; Wu, J.; Pang, X.; Wu, J.; Yang, X.; Tang, Y.; et al. MIF promotes perineural invasion through EMT in salivary adenoid cystic carcinoma. Mol. Carcinog. 2019, 58, 898–912.
- Xie, J.; Feng, Y.; Lin, T.; Huang, X.-Y.; Gan, R.-H.; Zhao, Y.; Su, B.-H.; Ding, L.-C.; She, L.; Chen, J.; et al. CDH4 suppresses the progression of salivary adenoid cystic carcinoma via E-cadherin co-expression. Oncotarget 2016, 7, 82961–82971.
- Jia, S.; Wang, W.; Hu, Z.; Shan, C.; Wang, L.; Wu, B.; Yang, Z.; Yang, X.; Lei, D. BDNF mediated TrkB activation contributes to the EMT progression and the poor prognosis in human salivary adenoid cystic carcinoma. Oral Oncol. 2015, 51, 64–70.
- Cavalcante, R.B.; Nonaka, C.F.W.; Rabenhorst, S.H.B.; Miguel, M.C.D.C.; Pinto, L.P.; de Souza, L.B. Pleomorphic adenoma and adenoid cystic carcinoma of salivary glands: E-cadherin immunoexpression and analysis of the CDH1 -160C/A polymorphism. Arch. Oral Biol. 2017, 73, 48–54.
- Phattarataratip, E.; Kositkittiwanit, N.; Kajornkiatkul, P.; Yeunyong, P.; Ratanapitak, R. P120 catenin expression and its correlation with E-cadherin in salivary gland neoplasms. J. Oral Biol. Craniofacial Res. 2019, 9, 57–62.
- Van Der Wal, J.E.; Sgaramella, N.; Spaak, L.N.; Zborayova, K.; Nylander, K. High podoplanin and low E-cadherin levels correlate with better prognosis in adenoid cystic carcinoma. Clin. Exp. Dent. Res. 2019, 5, 350–355.
- Wu, J.; Li, Z.; Wang, H.; Yu, X.; Pang, X.; Wu, J.; Wang, S.; Zhang, M.; Yang, X.; Cao, M.; et al. Cathepsin B defines leader cells during the collective invasion of salivary adenoid cystic carcinoma. Int. J. Oncol. 2019, 54, 1233–1244.
- Westcott, J.M.; Prechtl, A.M.; Maine, E.A.; Dang, T.; Esparza, M.; Sun, H.; Zhou, Y.; Xie, Y.; Pearson, G.W. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J. Clin. Investig. 2015, 125, 1927–1943.
- Konen, J.; Summerbell, E.; Dwivedi, B.; Galior, K.; Hou, Y.; Rusnak, L.; Chen, A.; Saltz, J.; Zhou, W.; Boise, L.H.; et al. Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nat. Commun. 2017, 8, 15078.
- Krakhmal, N.V.; Zavyalova, M.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V. Cancer Invasion: Patterns and Mechanisms. Acta Nat. 2015, 7, 17–28.
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144.
- Ostrowska-Podhorodecka, Z.; McCulloch, C.A. Vimentin regulates the assembly and function of matrix adhesions. Wound Repair Regen. 2021, 29, 602–612.
- Caselitz, J.; Becker, J.; Seifert, G.; Weber, K.; Osborn, M. Coexpression of keratin and vimentin filaments in adenoid cystic carcinomas of salivary glands. Virchows Arch. 1984, 403, 337–344.
- Chomette, G.; Auriol, M.; Vaillant, J.M.; Kasai, T.; Okada, Y.; Mori, M. Heterogeneity and co-expression of intermediate filament proteins in adenoid cystic carcinoma of salivary glands. Pathol. Biol. 1991, 39, 110–116.
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368.
- D’Ardenne, A.J.; Kirkpatrick, P.; Wells, C.A.; Davies, J.D. Laminin and fibronectin in adenoid cystic carcinoma. J. Clin. Pathol. 1986, 39, 138–144.
- Dong, F.; Wang, X.; Zhang, P. Study of electron microscopy histochemistry and immunohistochemistry of extracellular matrix in adenoid cystic carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi 1997, 15, 306–307.
- Wegner, A.; Waśniewska, E.; Golusiński, W.; Golusiński, P. Assessment of extracellular matrix proteins (laminin and fibronectin) in adenoid cystic carcinoma of salivary gland using morphometric method. Rep. Pract. Oncol. Radiother. 2007, 12, 339–343.
- Liu, Y.; Song, J.; Zhang, J.; Yang, L.; Liu, Z.; Wang, X. BTB/POZ domain-containing protein 7 is inversely associated with fibronectin expression in salivary adenoid cystic carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 468–477.
- Tian, Z.; Wang, Y.; Zhang, W. Immunohistochemical study of basement membrane proteins expression in adenoid cystic carcinoma of salivary glands. Hua Xi Kou Qiang Yi Xue Za Zhi 1999, 17, 236–238,253.
- Cao, Z.-Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320.
- Jiang, Y.; Feng, X.; Zheng, L.; Li, S.-L.; Ge, X.-Y.; Zhang, J.-G. Thioredoxin 1 mediates TGF-β-induced epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Oncotarget 2015, 6, 25506–25519.




