Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lubos Danisovic | -- | 1403 | 2023-05-26 11:48:30 | | | |
| 2 | Fanny Huang | Meta information modification | 1403 | 2023-05-28 03:01:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ivanisova, D.; Bohac, M.; Culenova, M.; Smolinska, V.; Danisovic, L. Mesenchymal-Stromal-Cell-Conditioned Media. Encyclopedia. Available online: https://encyclopedia.pub/entry/44885 (accessed on 08 February 2026).
Ivanisova D, Bohac M, Culenova M, Smolinska V, Danisovic L. Mesenchymal-Stromal-Cell-Conditioned Media. Encyclopedia. Available at: https://encyclopedia.pub/entry/44885. Accessed February 08, 2026.
Ivanisova, Dana, Martin Bohac, Martina Culenova, Veronika Smolinska, Lubos Danisovic. "Mesenchymal-Stromal-Cell-Conditioned Media" Encyclopedia, https://encyclopedia.pub/entry/44885 (accessed February 08, 2026).
Ivanisova, D., Bohac, M., Culenova, M., Smolinska, V., & Danisovic, L. (2023, May 26). Mesenchymal-Stromal-Cell-Conditioned Media. In Encyclopedia. https://encyclopedia.pub/entry/44885
Ivanisova, Dana, et al. "Mesenchymal-Stromal-Cell-Conditioned Media." Encyclopedia. Web. 26 May, 2023.
Copy Citation
Despite significant advances in biomedical research, osteochondral defects resulting from injury, an autoimmune condition, cancer, or other pathological conditions still represent a significant medical problem. Cell-based therapies and tissue engineering have gradually become promising alternatives. They combine the use of different types of cells and biomaterials to induce regeneration processes or replace damaged osteochondral tissue. One of the main challenges of this approach before clinical translation is the large-scale in vitro expansion of cells without changing their biological properties, while the use of conditioned media which contains various bioactive molecules appears to be very important.
conditioned medium
mesenchymal stem cells
1. Introduction
Diseases of the musculoskeletal system need to be strictly managed daily in clinical medicine. Affecting patients of all age groups, these pathologies significantly impact an individual’s health, as well as their psychical, social, and economic status [1]. Acute traumas, autoimmune diseases, or tumors heavily affect both bone and cartilage tissues. If treated unproperly, patients may be severely disabled [2]. The physiological regeneration of cartilage and bone is a complex process that involves tight cooperation between cellular and molecular agents near the affected site [3]. Continuous remodeling, as a direct response to physiological or pathological stimuli, is typical for osteochondral tissue regeneration [4]. In the case of bone regeneration, strict coordination between bone resorption provided by osteoclasts and new tissue formation arranged by osteoblasts needs to be maintained to keep the required integrity and functionality of the bone [5]. Cartilage regeneration is affected by the fact that it is an avascular and aneural tissue. Moreover, resident chondrocytes, which are responsible for the production of important extracellular matrix (ECM) components, have a very low proliferative capacity, and, therefore, regeneration is considerably slowed down and, in many cases, it is not possible to achieve a satisfactory result.
Fractures represent the most common injuries of bone tissue, and the healing process involves inflammatory, proliferative, and remodeling stages [6]. Effective blood supply to the affected area is crucial for the overall process of new bone formation and is related to the recruitment of adjacent stem cells that can differentiate into blood vessels [7]. Although the tissue’s propensity for self-repair is high, lower-quality tissue can still be formed and negatively affect patients´ life. Limited vascularization of the cartilage is one of the reasons why its intrinsic self-healing capacity is so restricted and why it is practically unable to repair [8]. Currently used surgical techniques often lead to the formation of scar tissue [9]. Novel approaches provided by tissue engineering and regenerative medicine might offer promising alternatives for the effective healing of even large defects affecting bone, as well as cartilage. Due to their differential healing capacity, mesenchymal stromal cells (MSCs) have been intensively studied concerning osteochondral regeneration [10].
Many in vitro studies have investigated their potential when applied as stem-cell therapy or combined with scaffolds [11]. Long-running and detailed investigations of MSCs have helped us to elucidate complex processes which are responsible for their biological actions. It is accepted that MSCs operate via the secretion of active molecules such as proteins, lipids, cytokines, mRNAs, and growth factors, which seem to have a direct effect on tissue regeneration [12]. To minimize the risks for patients, attention is currently drawn to the evaluation of the effect of the MSC-conditioned medium (MSC-CM) as a potential candidate for cell-free therapy (Figure 1). In addition, various studies that focused on clinical MSC application have suggested that their regenerative properties are not related to the proximity of the applied cells to the targeted tissue [13]. Moreover, it was also described that the applied cells could not maintain their lifespan for a long time, and, therefore, their effect needed to be provided by secreted bioactive molecules [13]. The MSC secretome presents a package of bioactive agents secreted into extracellular space [14]. It can be easily obtained with collection of CM and various techniques and protocols have been already applied for its analysis [15].
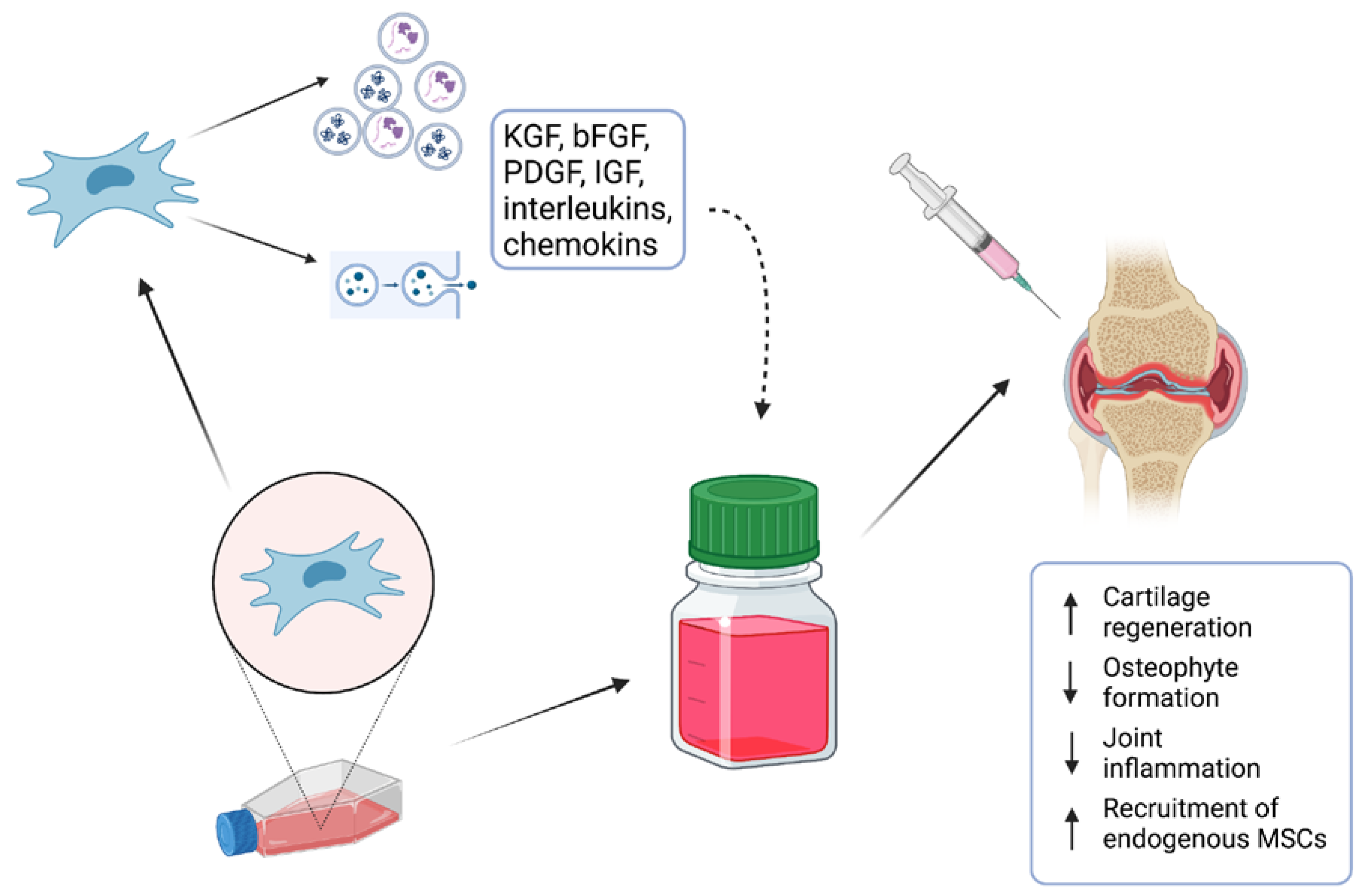
Figure 1. MSC-conditioned medium (MSC-CM) containing various proteins, lipids, cytokines, mRNAs, and growth factors is a potential candidate for cell-free therapy of osteochondral defects.
2. Preparation of MSC-Conditioned Medium
MSC-CM is a type of cell culture medium that has been conditioned or modified by the paracrine action of MSCs. This medium contains various growth factors, cytokines, and other molecules secreted by the MSCs and can be used for various purposes, such as promoting cell growth and differentiation, studying cell signaling pathways, and evaluating the therapeutic potential of MSCs [16]. The most common sources of stem cells which are used for MSC-CM “fabrication” are bone marrow MSCs, adipose tissue MSCs, and dental pulp MSCs [17][18].
The first step of MSC-CM production is the isolation and in vitro expansion of MSCs under static or dynamic conditions to obtain a suitable number of cells before starting the conditioning process. Recently, a fully closed, automated, and GMP-compliant cell expansion system (e.g., CliniMACS Prodigy®) was developed which can be used to allow the large-scale production of MSCs. After a precise characterization of MSCs, culture media are replaced by “starving” serum-free basal media. In this step, MSCs start to secrete various bioactive molecules and extracellular vesicles. In some cases, MSCs are cultured under specific conditions, such as low oxygen tension or the addition of specific growth factors, to encourage the secretion of bioactive molecules into the media. After a certain period of time, typically 24–48 h, the conditioned media are harvested and filtered to remove any cell debris. Afterward, MSC-CM can be concentrated to increase the concentration of bioactive molecules. This can be carried out using various methods, such as centrifugation or ultrafiltration. The final step is the quality control of the fabricated MSC-CM. MSC-CM is usually analyzed for the presence of specific bioactive molecules, such as growth factors and cytokines, using techniques such as ELISA or mass spectrometry [19][20].
3. Scaffold Pretreating Using MSC-Conditioned Medium
Within the fields of tissue engineering and regenerative medicine, cells, scaffolds, and growth factors create the main pillars for effective tissue regeneration. Various studies confirmed that the application of scaffolds could enhance bone healing when compared to only SC application [21]. Moreover, recent studies suggested that pretreatment of the scaffolds with MSC-CM might have provided even better results. Scaffold pretreating using MSC-conditioned medium refers to a technique that involves treating a scaffold material with a conditioned medium derived from MSCs before seeding cells onto the scaffold. MSCs are known to secrete various growth factors and cytokines that can stimulate cellular proliferation, migration, and differentiation. By pretreating the scaffold with MSC-CM, the scaffold can be “primed” with these growth factors and cytokines, providing a more favorable environment for seeded cells to adhere, proliferate, and differentiate [22].
Garcìa-Ruìz and colleagues applied MSC-CM from bone marrow MSCs and used it to improve the biological properties of a 3D-printed composite scaffold [23]. The results showed that cells seeded on pretreated scaffolds could attach and proliferate better on the scaffold´s surface. In addition, chondrogenic differentiation was promoted at a higher rate as well, making this approach a promising strategy used for osteochondral regeneration. A similar technique was utilized in the following studies to repair bone tissue under in vivo conditions [5][12]. Seeded scaffolds affected by MSC-CM repaired rat calvarial defects more successfully. These outcomes were also supported by in vitro results which revealed a higher expression of osteogenic genes in applied cells. In a more recent study performed by Chang and colleagues, tissue-engineered bones were fabricated with a combination of demineralized bone matrix and MSCs to treat large segmental bone defects. They also used the pretreatment of scaffolds with MSC-CM. Their results demonstrated that MSC-CM containing significant concentrations of various growth factors had a positive effect on MSC migration, proliferation, and osteogenic differentiation [24].
The improved bone-healing capacity of MCS-CM related to electrical stimuli and a 3D culture system was investigated in a study carried out by Hwang and colleagues [25]. MSCs were seeded on collagen sponges and constructs were/were not exposed to electric stimuli. Combining the mentioned culture techniques seemed to significantly improve inflammatory-mediated bone loss repair. Ogata and coworkers used atelocollagen in combination with human MSC-CM to treat bone defects. They showed an enhanced migration of endogenous osteoprogenitor cells and accelerated bone regeneration [26]. More recently, Dilogo and coworkers conducted a large animal study investigating the effect of hydroxyapatite and MSC-CM on the treatment of critical-sized bone defects. They applied MSC-CM alone or with the supplementation of BMP-2. They demonstrated the most significant effect on total callus formation in the group treated with MSC-CM and BMP-2, while the osseous area was found to be highest in the MSC-CM group [27].
References
- Jagodzinski, M.; Haasper, C. General Principles for the Regeneration of Bone and Cartilage. In Mesenchymal Stem Cells—Basics and Clinical Application II; Weyand, B., Dominici, M., Hass, R., Jacobs, R., Kasper, C., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 130, pp. 69–88. ISBN 978-3-642-37943-7.
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268.
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone Regeneration and Stem Cells. J. Cell. Mol. Med. 2011, 15, 718–746.
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66.
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.; Mazzon, E.; Trubiani, O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Int. J. Mol. Sci. 2018, 19, 329.
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone Injury and Fracture Healing Biology. Biomed. Environ. Sci. 2015, 28, 57–71.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54.
- Huang, K.; Li, Q.; Li, Y.; Yao, Z.; Luo, D.; Rao, P.; Xiao, J. Cartilage Tissue Regeneration: The Roles of Cells, Stimulating Factors and Scaffolds. CSCR 2018, 13, 547–567.
- Gomoll, A.H.; Minas, T. The Quality of Healing: Articular Cartilage: Articular Cartilage. Wound Repair. Regen. 2014, 22, 30–38.
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492.
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-Derived Mesenchymal Stem Cells: Biology and Potential Applications. In Mesenchymal Stem Cells—Basics and Clinical Application I; Weyand, B., Dominici, M., Hass, R., Jacobs, R., Kasper, C., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 129, pp. 59–71. ISBN 978-3-642-35670-4.
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochem. Mosc. 2019, 84, 1375–1389.
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467.
- Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals 2020, 13, 31.
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656.
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886.
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T.-H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 21, 49.
- Joseph, A.; Baiju, I.; Bhat, I.A.; Pandey, S.; Bharti, M.; Verma, M.; Pratap Singh, A.; Ansari, M.M.; Chandra, V.; Saikumar, G.; et al. Mesenchymal Stem Cell-conditioned Media: A Novel Alternative of Stem Cell Therapy for Quality Wound Healing. J. Cell. Physiol. 2020, 235, 5555–5569.
- Thalakiriyawa, D.S.; Jayasooriya, P.R.; Dissanayaka, W.L. Regenerative Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles. CMM 2022, 22, 98–119.
- Lichte, P.; Pape, H.C.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for Bone Healing: Concepts, Materials and Evidence. Injury 2011, 42, 569–573.
- Wang, S.-H.; Lee, S.-P.; Yang, C.-W.; Lo, C.-M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441.
- García-Ruíz, J.; Díaz Lantada, A. 3D Printed Structures Filled with Carbon Fibers and Functionalized with Mesenchymal Stem Cell Conditioned Media as In Vitro Cell Niches for Promoting Chondrogenesis. Materials 2017, 11, 23.
- Chang, Z.; Xing, J.; Yu, X. Construction and Evaluation of a Novel Tissue-engineered Bone Device. Exp. Ther. Med. 2021, 22, 1166.
- Hwang, S.J.; Cho, T.H.; Lee, B.; Kim, I.S. Bone-Healing Capacity of Conditioned Medium Derived from Three-Dimensionally Cultivated Human Mesenchymal Stem Cells and Electrical Stimulation on Collagen Sponge. J. Biomed. Mater. Res. 2018, 106, 311–320.
- Ogata, K.; Osugi, M.; Kawai, T.; Wakayama, Y.; Sakaguchi, K.; Nakamura, S.; Katagiri, W. Secretomes of Mesenchymal Stem Cells Induce Early Bone Regeneration by Accelerating Migration of Stem Cells. J. Oral. Maxillofac. Surg. Med. Pathol. 2018, 30, 445–451.
- Dilogo, I.; Fiolin, J.; Canintika, A.; Pawitan, J.; Luviah, E. The Effect of Secretome, Xenogenic Bone Marrow-Derived Mesenchymal Stem Cells, Bone Morphogenetic Protein-2, Hydroxyapatite Granule and Mechanical Fixation in Critical-Size Defects of Rat Models. ABJS 2021, 10, 17–22.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
600
Revisions:
2 times
(View History)
Update Date:
28 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




