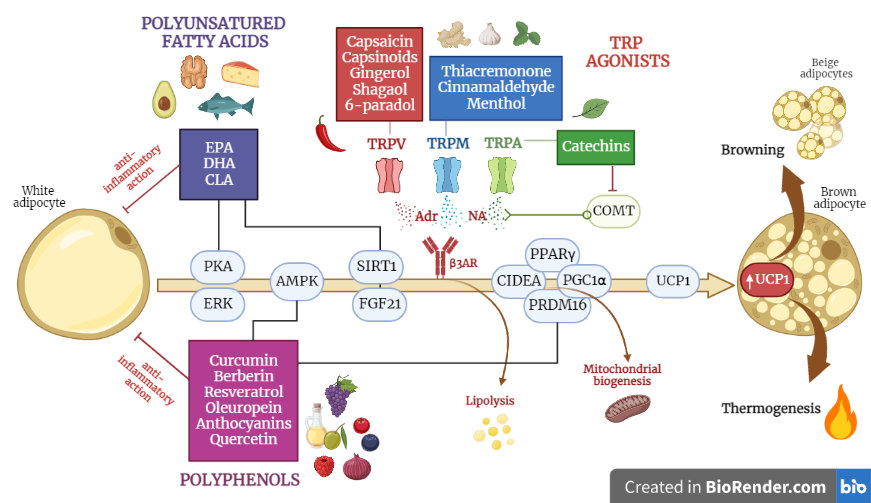
1. Polyunsatured Fatty Acids
Fish oil is rich in the polyunsaturated fatty acids (PUFA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which stimulate thermogenesis in BAT
[1][2][3]. In the early 2000s, several studies in mouse models compared the effects of a diet enriched with
n-3 PUFAs and a diet rich in
n-6 PUFAs in order to study their impact on BAT. It turned out that fats from the
n-3 series were much more effective in activating BAT than the
n-6 series
[4]. Therefore, subsequent research focused on the effects of
n-3 PUFAs, especially EPA, and confirmed its anti-obesogenic effect, revealing that it can promote the browning of WAT. Rats fed with a supplementation of fish oil at a 20% EPA for 4 weeks showed an increased mRNA level of UCP1 in WAT
[5]. Another study showed that supplementation with 27.5% fish oil, administered for one month, stimulates SNS-mediated mitochondrial and thermogenic activity in rats
[6]. A diet enriched with a mixture of EPA and DHA increases the expression of UCP1 in BAT and reduces adipose accumulation via the induction of marked, non-shivering thermogenesis
[7][8]. Further implications emerged from a study conducted in 2016, which revealed that a high-fat diet (HFD), enriched with fish oil (12% of total lipids) and administered to mice for 8 weeks, modulates BAT activation via epigenetic regulation
[9]. EPA promotes both the adipogenesis of mature brown adipocytes
[10] and the differentiation of pre-adipocytes into beige adipocytes, within WAT stores, particularly in the inguinal WAT
[8]. It is important to note that the conversion of mature white adipocytes to beige adipocytes was achieved via supplementation with EPA in human adipocyte cultures
[11] and that the same effect was not observed in rodents
[3].
2. Capsaicin and Capsinoids
Capsaicin is an alkaloid found in chilli peppers that is mainly concentrated in the seeds of the fruit and is the main component responsible for the spicy flavour. Capsaicin has a role in preventing the obesogenic effects of diet, due to its ability to increase energy expenditure by activating BAT
[12]. Capsaicin implements BAT function via several signalling pathways, primarily via the activation of Transient Receptor Potential Vanilloid 1 channels (TRPV1), which stimulate the central nervous system to produce catecholamines involved in thermogenesis. Capsaicin seems to regulate the epigenetic expression of the transcription factors involved in WAT browning
[13][14]. Important evidence has also emerged from studies on humans: Yoneshiro et al.
[15] examined the acute effect of an oral ingestion of a single dose of capsinoids (9 mg) on energy expenditure, in relation to BAT activity in humans; this was measured by 18F-FDG PET, a classical imaging technique used to visualize BAT activation. The ingestion of capsinoids resulted in a 3-fold increase in energy expenditure in the BAT-positive group compared to the BAT-negative group. Furthermore, the chronic intake of capsinoids (9 mg/day in capsule form) for 6 weeks promoted BAT activity and reduced fat mass after cold exposure, even in human subjects with low BAT activity. In 2018, Camps et al.
[16] tested the same treatment used in mice in 2016
[17] on humans. They gave a group of normal-weight individuals a dose of capsinoids (12 mg), combined with exposure to cold (14.5 °C). The subjects underwent, before and after exposure, both 18F-FDG PET and indirect calorimetry, in order to assess the total energy expenditure. The results showed that capsinoids increased energy expenditure in BAT-positive participants and, when combined with cold, increased fat oxidation, improved insulin sensitivity and increased HDL-cholesterol
[18][19].
3. Green Tea Catechins
Green tea consumption is associated with weight loss and the modulation of fat metabolism and energy expenditure
[20][21]. Catechin supplementation for 8 weeks reduced the mass of perirenal WAT and increased the expression of mRNA coding for UCP1 in BAT, compared to the control group in rats
[22]. Yan and al.
[23] showed that the oral administration of catechins (100 mg/kg body weight) for 4 weeks significantly reduced the total fat mass (subcutaneous and visceral) and liver size in rats fed with HFD. No browning of the WAT was observed, but fatty acid oxidation in the BAT increased twofold
[23]. Several experiments testing the effects of catechin supplementation on rodents were also conducted on humans. The habitual intake of green tea (>300 mg catechins/day) was found to be as effective as in rats in terms of reducing body weight and preventing weight regain
[24]. Similar results were obtained in a study on subjects with metabolic syndrome, where catechin supplementation additionally improved the lipid profile and hypertension
[25]. The consumption of 300 mg/day of Epigallocatechin Gallate (EGCG) or 200 mg of caffeine
[26] for 3 days in subjects with obesity increased postprandial fat oxidation, but not the total energy expenditure
[27]. A 12-week treatment with green tea extracts containing 583 mg catechins resulted in a small but significant reduction in body fat (3–5%) in healthy, normal-weight subjects
[28]. A more recent study
[29] suggested that catechins also act as inhibitors of catechol-O-methyltransferases (COMT), enzymes that degrade catecholamines. The inhibition of COMTs results in the increased metabolism of catecholamines, including noradrenaline, which, as the researchers have seen, is involved in thermogenesis and fat oxidation processes.
4. Curcumin
Curcumin is the most abundant and active element within the turmeric rhizome; it is a widely studied polyphenol with antioxidant and anti-inflammatory properties, also known for its anti-obesity effects
[30]. Isolated WAT cells of obese rats, in response to treatment with 20 μM of curcumin for 6–8 days, showed an increase in the thermogenic markers of BAT and in the hormone-sensitive lipase (HSL), which is responsible for triglyceride mobilization and lipolysis in WAT. These results suggest curcumin’s role in improving the lipid profile in an obese condition
[31]. The administration of high doses of curcumin (45 mg/kg body weight) causes an increase in energy expenditure via the induction of mitochondrial biogenesis in mice
[32]. Experimental evidence on humans is still limited. However, one significant study in the literature stands out; this was conducted in 2019 on 60 participants
[33][34], with the aim of assessing the effects of curcumin supplementation on cardiovascular risk factors among overweight adolescents. It was a randomized controlled trial involving 60 girls aged 13–18 years, randomly assigned to the intervention or control group. The intervention group was supplemented with 500 mg of curcumin (in bioavailable form) daily for 10 weeks, while the control group received a placebo. At the same time, they were asked to undergo a mild weight loss or weight maintenance diet, depending on the degree of overweight or obesity. The results revealed that curcumin supplementation induced significant improvements compared to the control group, such as a reduction in the body mass index, a reduction in waist circumference and hip circumference, and reduction in the triglyceride/HDL ratio; meanwhile, it induced an increase in the HDL cholesterol. However, clinical studies on a larger population and with a longer duration are needed to confirm the results of these studies
[33][34] and to test the WAT darkening effects observed in rodents also in humans.
5. Resveratrol
Resveratrol is a polyphenol naturally contained in grape skins and other vegetables such as red fruits, cocoa and peanuts
[35]. The supplementation of 30 mg/kg body weight of resveratrol in mouse models for 8 weeks showed a significant reduction in fat mass, plasma glucose concentrations and total cholesterol, compared to the control group
[36]. There was also evidence of increased UCP1 expression in both BAT and skeletal muscle
[37]. This is attributed, at least in part, to the ability of resveratrol to activate upstream AMPK, which promotes the production of PGC1α and Sirtuin 1 (SIRT1); these, in turn, promote mitochondrial biogenesis and WAT browning
[38]. The effect of resveratrol on the neo-formation of beige adipocytes is thought to be mediated by the phosphorylation of AMPK, as the deletion of this protein in mice abolishes the browning effects of resveratrol
[39]. A recent study
[40] revealed the additional signalling pathways exerted by resveratrol. The results reported regarding the cell cultures and animal models suggest that high doses of resveratrol (100 and 200 μM) induce epigenetic regulatory mechanisms. Polyphenol can up-regulate the expression of the genes that code for the proteins involved in WAT adipogenesis, such as fatty acid synthase (FAS), the sterol response element-binding protein (SREBP1), lipoprotein lipase (LPL) and hormone-sensitive lipase (HSL)
[41]. Overall, resveratrol oversees antiadipogenic and anti-inflammatory effects, which are dependent and independent of BAT activation; however, evidence supporting its thermogenic effects in humans is currently lacking. This is due to the poor bioavailability of polyphenolic compounds
[42], so it is difficult to achieve effective doses that are able to stimulate BAT through food alone. Diet alone is not sufficient in order to mimic the efficacy of resveratrol observed in rodents.
6. Berberine
Berberine is an alkaloid compound found in the roots, rhizomes and bark of certain plants of the genus Berberis, primarily known for its anti-cancer activity
[43]. Zhang et al.
[44] proved that the intraperitoneal administration of berberine, administered at 5 mg/kg/day for 4 weeks in obesity-induced mice, played a key role in increasing energy expenditure and the mobilization of lipids. More specifically, the thermogenic effects attributed to berberine concerned the increase in the mitochondrial content and thermogenic markers in BAT (UCP1, PGC1α, Cidea). The increase in BAT was detected via PET-CT, while the increase in the resting energy expenditure was quantified by measuring the oxygen consumption (VO
2) and carbon dioxide release (VCO
2) before and after treatment. The total expenditure increased by 20%.
An interesting finding, which did not emerge from studies on the other phytochemical compounds, is that this treatment caused a significant reduction in the respiratory quotient, suggesting that berberine causes a shift in cell metabolism, taking energy from the oxidation of fatty acids, rather than from carbohydrates. Furthermore, mice berberine not only stimulates BAT activity but also induces the browning of the inguinal WAT, probably via the phosphorylation of AMPK, which leads to the increased expression of UCP1 and PGC1α
[45]. An in vitro study was recently conducted on human preadipocytes, and it was found that the thermogenic effect induced by berberine on WAT depends on the recruitment of the AMPK-PRDM16 axis: the activation of AMPK could lead to DNA demethylation and thus upregulate the expression of PRDM16, which acts as a master regulator of the transdifferentiation of white adipocytes into beige adipocytes; this occurs via the direct modulation of the transcription factors PPARγ and PGC1α
[46][47].
7. Other Nutraceutical Compounds
Many other dietary polyphenolic compounds have been found, in rodent studies, to influence BAT thermogenesis and WAT browning: oleuropein, anthocyanins, quercetin, and structural analogues of capsaicin (i.e., menthol, cinnamaldehyde, allyl and benzyl isothiocyanates, which are the spicy elements found in mustard and wasabi, and thiacremonone). Some of these compounds have also shown promising results in humans.
7.1. Oleuropein
Oleuropein is the main polyphenol contained in olive leaves and fruits, and is responsible for the pungent and bitter taste of raw olives. Olive leaf extract (3 mg of oleuropein, injected intravenously in mice for 7 weeks) increases UCP1 content in BAT by activating SIRT1, PPARγ and PGC1α. In addition, it stimulates the secretion of adrenalin and noradrenalin via the activation of TRP channels
[48]. Oleuropein aglycone (the absorbed form of oleuropein) appears to be able to attenuate diet-induced obesity by supporting the expression of thermogenic genes and genes related to mitochondrial biogenesis in the BAT of overfed mice
[49], and to promote the browning of adipose tissue via mesenchymal stem cells in humans
[50].
7.2. Anthocyanins
Anthocyanins are the polyphenols mainly contained in red fruits, grapes, black soy, and red beans. Anthocyanins are known first and foremost for their strong antioxidant power, but they have also been re-evaluated for their thermogenic action in stimulating adrenalin secretion and energy metabolism in humans; meanwhile, in rodents, they exert an anti-obesity action that is related to BAT activation
[51][52]. In humans, the intake of 150 mg/day of an extract of Aronia melanocarpa, a particular type of blueberry rich in anthocyanins, procyanidins and other flavonoids, increases the surface body temperature and plasma adrenalin levels, suggesting that it has a stimulating effect on the SNS
[53]. Numerous studies have also shown that long-term treatment with cyanidin-3-glucoside, which is found in raspberry and mulberry extract, increases UCP1 expression and mitochondrial biogenesis during the adipogenic differentiation of brown and white preadipocytes
[54][55]. Similarly, black soybean peel extract upregulates the expression of thermogenic genes in BAT, induces WAT browning and increases the lipid respiration quotient, thus preventing visceral fat accumulation in mice on a hyperlipidic diet
[56].
7.3. Quercetin
Quercetin is a flavonoid contained in a wide variety of fruits (apples, grapes, olives, citrus fruits, berries) and vegetables (tomatoes, onions, broccoli, and capers). It has a thermogenic effect in mice and an ability to modulate the gut microbiota in mice. Obese mice fed a HFD supplemented with 1% quercetin for 16 weeks lost weight and had reduced plasma cholesterol levels, compared to mice fed a HFD alone
[57]. The improvement in obesity is explained by the ability of quercetin to increase the expression of thermogenic genes in BAT (UCP1, PGC1α. FGF21), as well as and genes coding for β-adrenergic receptors and AMPK; this results in increased non-excitation thermogenesis. The increase in AMPK also suggests that quercetin may predispose these signalling pathways to WAT browning
[58]. Other studies point out the flavonoid’s ability to modulate the intestinal microbial composition of mice. Quercetin reduces the ratio of Firmicutes to Bacteroidetes in the microbiota of HFD-fed mice, improving the obesity-related dysbiosis picture. In fact, a eubiotic microbiota allows greater energy extraction from the diet via the increased production of short-chain fatty acids, which have been found in the faeces of mice supplemented with quercetin
[57].
7.4. Analogues of Capsaicin
Structural analogues of capsaicin include menthol, which is a cyclic monoterpene alcohol obtained from peppermint, the activator of TRPM8 receptors
[59][60], cinnamaldehyde, a pungent compound that increases UCP1 expression in human white adipocytes
[61] and is found in cinnamon, allyl and benzyl isothiocyanates, which are spicy elements found in mustard, ginger and wasabi (Japanese horseradish), and finally, thiacremonone, a sulphur compound isolated in garlic
[62]. The compounds just mentioned can activate TRPV1 and TRPA1 channels (also belonging to the TRP receptor family, but activated by cold, mechanical stimuli and cooling nutritional compounds), which modulate thermoregulation. The combination of sub-effective doses of capsaicin, cinnamaldehyde and menthol induce the “brite” phenotype in the differentiation of the 3T3-L1 cells and subcutaneous white adipose tissue of HFD-fed obese mice
[63]. The intervention prevented adipose tissue hypertrophy and weight gain, and enhanced the thermogenic potential, mitochondrial biogenesis, and overall activation of brown adipose tissue. These changes, observed in vitro as well as in vivo, were linked to the increased phosphorylation of kinases, AMPK and ERK. In the liver, this combination treatment enhanced insulin sensitivity, improved the gluconeogenic potential and lipolysis, prevented fatty acid accumulation and enhanced glucose utilization. Finally, it is worth mentioning that gingerol, shogaol and 6-paradol, which are all contained in ginger root, activate TRPV1 channels
[64][65][66]. An interesting study in humans looked at a type of ginger native to West Africa (black ginger), from which the extract Kaempferia parviflora is derived. The administration of 100 mg/day of this extract appears to increase energy expenditure in BAT-positive subjects, and the activation of brown adipocytes is detectable via FDG-PET after exposure to cold
[67].