
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Meng Zheng | -- | 1491 | 2023-05-25 05:24:10 | | | |
| 2 | Lindsay Dong | Meta information modification | 1491 | 2023-05-25 07:14:54 | | |
Video Upload Options
Highly bio-compatible organic semiconductors are widely used as biosensors, but their long-term stability can be compromised due to photo-degradation and structural instability. To address this issue, scientists have developed organic semiconductor nanoparticles (OSNs) by incorporating organic semiconductors into a stable framework or self-assembled structure. OSNs have shown excellent performance and can be used as high-resolution biosensors in modern medical and biological research. They have been used for a wide range of applications, such as detecting small biological molecules, nucleic acids, and enzyme levels, as well as vascular imaging, tumor localization, and more. In particular, OSNs can simulate fine particulate matters (PM2.5, indicating particulate matter with an aerodynamic diameter less than or equal to 2.5 μm) and can be used to study the biodistribution, clearance pathways, and health effects of such particles.
1. Introduction
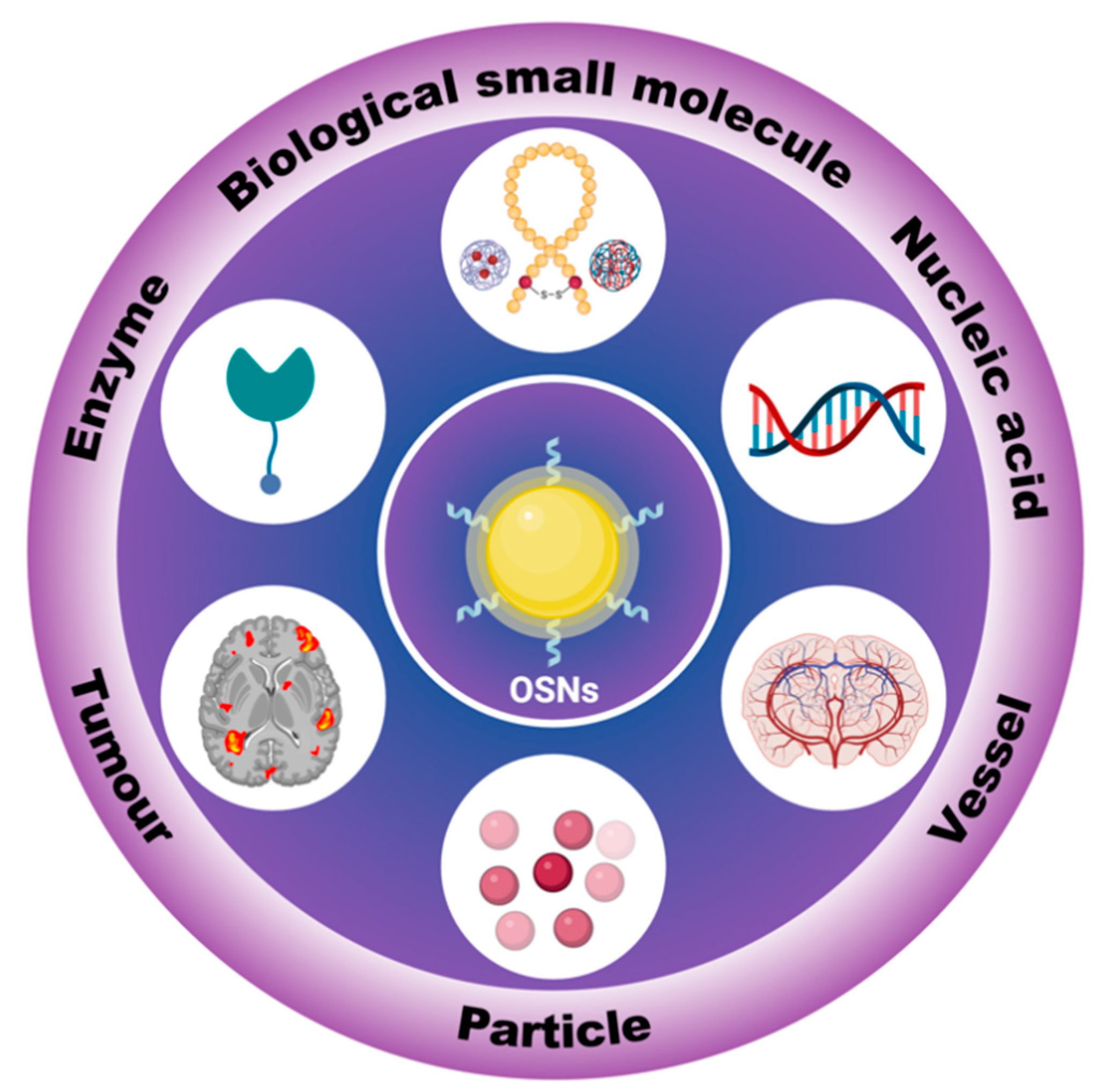
2. In Vitro Tracking
2.1. Biological Small Molecules Tracking
2.2. Enzyme Concentration Measurement
2.3. Nucleic Acid Concentration Measurement
3. In Vivo Tracking
3.1. Tumor Localization
3.2. Blood Vessel Imaging
3.3. Particles Tracking
4. Conclusions
References
- Gong, S.; Qin, A.; Zhang, Y.; Li, M.; Chen, X.; Liang, Y.; Xu, X.; Wang, Z.; Wang, S. A New Ratiometric AIE Fluorescent Probe for Detecting Cysteine in Food Samples and Imaging in the Biological System. Food Chem. 2023, 400, 134108.
- Bao, B.; Ma, M.; Zai, H.; Zhang, L.; Fu, N.; Huang, W.; Wang, L. Conjugated Polymer Nanoparticles for Label-Free and Bioconjugate-Recognized DNA Sensing in Serum. Adv. Sci. 2015, 2, 1400009.
- Lin, H.; Bai, H.; Yang, Z.; Shen, Q.; Li, M.; Huang, Y.; Lv, F.; Wang, S. Conjugated Polymers for Biomedical Applications. Chem. Commun. 2022, 58, 7232–7244.
- Li, J.; Jiang, R.; Wang, Q.; Li, X.; Hu, X.; Yuan, Y.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Semiconducting Polymer Nanotheranostics for NIR-II/Photoacoustic Imaging-Guided Photothermal Initiated Nitric Oxide/Photothermal Therapy. Biomaterials 2019, 217, 119304.
- Gao, H.; Kam, C.; Chou, T.Y.; Wu, M.-Y.; Zhao, X.; Chen, S. A Simple yet Effective AIE-Based Fluorescent Nano-Thermometer for Temperature Mapping in Living Cells Using Fluorescence Lifetime Imaging Microscopy. Nanoscale Horiz. 2020, 5, 488–494.
- Zhou, L.; Zhang, X.; Dong, Y.; Pan, Y.; Li, J.; Zang, Y.; Li, X. A Tandemly Activated Fluorescence Probe for Detecting Senescent Cells with Improved Selectivity by Targeting a Biomarker Combination. ACS Sens. 2022, 7, 1958–1966.
- Wang, R.; Zhou, L.; Wang, W.; Li, X.; Zhang, F. In Vivo Gastrointestinal Drug-Release Monitoring through Second near-Infrared Window Fluorescent Bioimaging with Orally Delivered Microcarriers. Nat. Commun. 2017, 8, 14702.
- Xu, J.; Xiong, J.; Qin, Y.; Li, Z.; Pan, C.; Huo, Y.; Zhang, H. A Novel Quinolinyl-Tetraphenylethene-Based Fluorescence “Turn-on” Sensor for Zn2+ with a Large Stokes Shift and Its Applications for Portable Test Strips and Biological Imaging. Mater. Chem. Front. 2020, 4, 3338–3348.
- Enbanathan, S.; Iyer, S.K. A Novel Phenanthridine and Terpyridine Based D-π-A Fluorescent Probe for the Ratiometric Detection of Cd2+ in Environmental Water Samples and Living Cells. Ecotoxicol. Environ. Saf. 2022, 247, 114272.
- Jiang, N.; Gong, X.; Zhong, T.; Zheng, Y.; Wang, G. A Highly Selective and Sensitive “Turn-on” Fluorescent Probe for Rapid Recognition and Detection of Cu2+ in Aqueous Solution and in Living Cells. J. Mol. Struct. 2020, 1219, 128573.
- Li, Q.; Ding, Q.; Li, Y.; Zeng, X.; Liu, Y.; Lu, S.; Zhou, H.; Wang, X.; Wu, J.; Meng, X.; et al. Novel Small-Molecule Fluorophores for in Vivo NIR-IIa and NIR-IIb Imaging. Chem. Commun. 2020, 56, 3289–3292.
- Fortibui, M.M.; Jang, M.; Lee, S.; Ryoo, I.-J.; Ahn, J.S.; Ko, S.-K.; Kim, J. Near-Infrared Fluorescence Probe for Specific Detection of Acetylcholinesterase and Imaging in Live Cells and Zebrafish. ACS Appl. Bio Mater. 2022, 5, 2232–2239.
- Bhaskar, S.; Srinivasan, V.; Ramamurthy, S.S. Nd2O3-Ag Nanostructures for Plasmonic Biosensing, Antimicrobial, and Anticancer Applications. ACS Appl. Nano Mater. 2023, 6, 1129–1145.
- Chen, H.; Cheng, Z.; Zhou, X.; Wang, R.; Yu, F. Emergence of Surface-Enhanced Raman Scattering Probes in Near-Infrared Windows for Biosensing and Bioimaging. Anal. Chem. 2022, 94, 143–164.
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, S.T.H.; Lam, J.W.Y.; Kwok, R.T.K.; Qian, J.; Tang, B.Z. Design of AIEgens for Near-Infrared IIb Imaging through Structural Modulation at Molecular and Morphological Levels. Nat. Commun. 2020, 11, 1255.
- Albani, J.R. Principles and Applications of Fluorescence Spectroscopy, 1st ed.; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-1-4051-3891-8.
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of Superior Phototheranostic Agents Guided by Jablonski Diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234.
- Han, W.; Du, Y.; Song, M.; Sun, K.; Xu, B.; Yan, F.; Tian, W. Fluorescent Nanorods Based on 9,10-Distyrylanthracene (DSA) Derivatives for Efficient and Long-Term Bioimaging. J. Mater. Chem. B 2020, 8, 9544–9554.
- Liu, S.; Zhu, Y.; Wu, P.; Xiong, H. Highly Sensitive D–A–D-Type Near-Infrared Fluorescent Probe for Nitric Oxide Real-Time Imaging in Inflammatory Bowel Disease. Anal. Chem. 2021, 93, 4975–4983.
- Deng, S.; Li, L.; Zhang, J.; Wang, Y.; Huang, Z.; Chen, H. Semiconducting Polymer Dots for Point-of-Care Biosensing and In Vivo Bioimaging: A Concise Review. Biosensors 2023, 13, 137.
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668.
- Zhao, Z.; Bi, X.; Mao, W.; Xu, X. A Novel HPQ-Based Turn-on Fluorescent Probe for Detection of Fluoride Ions in Living Cells. Tetrahedron Lett. 2017, 58, 4129–4132.
- Li, K.; Lyu, Y.; Huang, Y.; Xu, S.; Liu, H.-W.; Chen, L.; Ren, T.-B.; Xiong, M.; Huan, S.; Yuan, L.; et al. A de Novo Strategy to Develop NIR Precipitating Fluorochrome for Long-Term in Situ Cell Membrane Bioimaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2018033118.
- Li, B.; Chen, T.; Wang, Z.; Guo, Z.; Peña, J.; Zeng, L.; Xing, J. A Novel Cross-Linked Nanoparticle with Aggregation-Induced Emission Properties for Cancer Cell Imaging. J. Mater. Chem. B 2020, 8, 2431–2437.
- Yu, C.Y.Y.; Zhang, W.; Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIEgen for Nucleolus and Mitochondria Imaging with Organelle-Specific Emission. J. Mater. Chem. B 2016, 4, 2614–2619.
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414.
- Wang, Y.; Wu, W.; Liu, J.; Manghnani, P.N.; Hu, F.; Ma, D.; Teh, C.; Wang, B.; Liu, B. Cancer-Cell-Activated Photodynamic Therapy Assisted by Cu(II)-Based Metal–Organic Framework. ACS Nano 2019, 13, 6879–6890.
- Hu, F.; Mao, D.; Kenry; Wang, Y.; Wu, W.; Zhao, D.; Kong, D.; Liu, B. Metal–Organic Framework as a Simple and General Inert Nanocarrier for Photosensitizers to Implement Activatable Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1707519.
- Cai, X.; Mao, D.; Wang, C.; Kong, D.; Cheng, X.; Liu, B. Multifunctional Liposome: A Bright AIEgen-Lipid Conjugate with Strong Photosensitization. Angew. Chem. Int. Ed. 2018, 57, 16396–16400.
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951.
- Liang, B.; Hu, X.; Ding, Y.; Liu, M. Tumor-derived Exosomes in the PD-1/PD-L1 Axis: Significant Regulators as Well as Promising Clinical Targets. J. Cell. Physiol. 2021, 236, 4138–4151.
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530.
- Kuno, M.; Fromm, D.P.; Hamann, H.F.; Gallagher, A.; Nesbitt, D.J. Nonexponential “Blinking” Kinetics of Single CdSe Quantum Dots: A Universal Power Law Behavior. J. Chem. Phys. 2000, 112, 3117–3120.
- Smith, W.E.; Brownell, J.; White, C.C.; Afsharinejad, Z.; Tsai, J.; Hu, X.; Polyak, S.J.; Gao, X.; Kavanagh, T.J.; Eaton, D.L. In Vitro Toxicity Assessment of Amphiphillic Polymer-Coated CdSe/ZnS Quantum Dots in Two Human Liver Cell Models. ACS Nano 2012, 6, 9475–9484.
- Huang, H.; Zhang, X.; Hu, X.; Shao, Z.; Zhu, J.; Dai, L.; Man, Z.; Yuan, L.; Chen, H.; Zhou, C.; et al. A Functional Biphasic Biomaterial Homing Mesenchymal Stem Cells for in Vivo Cartilage Regeneration. Biomaterials 2014, 35, 9608–9619.
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898.
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity Assessment of Quantum Dots: From Cellular to Primate Studies. Chem. Soc. Rev. 2013, 42, 1236–1250.
- Hopkins, F.G.; Morgan, E.J. Some relations between ascorbic acid and glutathione. Biochem. J. 1936, 30, 1446–1462.
- Balendiran, G.K.; Dabur, R.; Fraser, D. The Role of Glutathione in Cancer. Cell Biochem. Funct. 2004, 22, 343–352.
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G.; Estrela, J.M.; Ortega, A.; et al. Enhanced Doubly Activated Dual Emission Fluorescent Probes for Selective Imaging of Glutathione or Cysteine in Living Systems. Anal. Chem. 2018, 90, 2648–2654.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem.-Biol. Interact. 2006, 160, 1–40.
- Mulay, S.V.; Kim, Y.; Choi, M.; Lee, D.Y.; Choi, J.; Lee, Y.; Jon, S.; Churchill, D.G.; Estrela, J.M.; Ortega, A.; et al. Glutathione in Cancer Biology and Therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181.
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12.
- McDonald, A.G.; Tipton, K.F. Enzyme Nomenclature and Classification: The State of the Art. FEBS J. 2022, febs.16274.
- Todorov, T.I.; Morris, M.D. Comparison of RNA, Single-Stranded DNA and Double-Stranded DNA Behavior during Capillary Electrophoresis in Semidilute Polymer Solutions. Electrophoresis 2002, 23, 1033–1044.
- Dahm, R. Discovering DNA: Friedrich Miescher and the Early Years of Nucleic Acid Research. Hum. Genet. 2008, 122, 565–581.
- Gregory, S.G.; Barlow, K.F.; McLay, K.E.; Kaul, R.; Swarbreck, D.; Dunham, A.; Scott, C.E.; Howe, K.L.; Woodfine, K.; Spencer, C.C.A.; et al. The DNA Sequence and Biological Annotation of Human Chromosome 1. Nature 2006, 441, 315–321.
- Zhang, C.-Y.; Yeh, H.-C.; Kuroki, M.T.; Wang, T.-H. Single-Quantum-Dot-Based DNA Nanosensor. Nat. Mater. 2005, 4, 826–831.
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459.
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2020, 6, 7–26.
- Jiang, Y.; Zhao, X.; Huang, J.; Li, J.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.; Duan, H.; et al. Transformable Hybrid Semiconducting Polymer Nanozyme for Second Near-Infrared Photothermal Ferrotherapy. Nat. Commun. 2020, 11, 1857.
- Zhen, X.; Xie, C.; Pu, K. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942.
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.; Gambhir, S.S.; Bao, Z.; Rao, J. Semiconducting Polymer Nanoparticles as Photoacoustic Molecular Imaging Probes in Living Mice. Nat. Nanotechnol. 2014, 9, 233–239.
- Zeng, W.; Wu, L.; Sun, Y.; Wang, Y.; Wang, J.; Ye, D. Ratiometric Imaging of MMP-2 Activity Facilitates Tumor Detection Using Activatable Near-Infrared Fluorescent Semiconducting Polymer Nanoparticles. Small 2021, 17, 2101924.
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626.
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A Graphene Quantum Dot Photodynamic Therapy Agent with High Singlet Oxygen Generation. Nat. Commun. 2014, 5, 4596.
- Lin, S.; Liu, C.; Han, X.; Zhong, H.; Cheng, C. Viral Nanoparticle System: An Effective Platform for Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 1728.
- Shivran, N.; Tyagi, M.; Mula, S.; Gupta, P.; Saha, B.; Patro, B.S.; Chattopadhyay, S. Syntheses and Photodynamic Activity of Some Glucose-Conjugated BODIPY Dyes. Eur. J. Med. Chem. 2016, 122, 352–365.
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-Photon Excitation Nanoparticles for Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6725–6741.
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-Based Nanoparticles Entrapping Water-Insoluble Photosensitizing Anticancer Drugs: A Novel Drug−Carrier System for Photodynamic Therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865.
- Citrin, D.; Lee, A.K.; Scott, T.; Sproull, M.; Ménard, C.; Tofilon, P.J.; Camphausen, K. In Vivo Tumor Imaging in Mice with near-Infrared Labeled Endostatin. Mol. Cancer Ther. 2004, 3, 481–488.
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.M.S.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational Design of a Water-Soluble NIR AIEgen, and Its Application in Ultrafast Wash-Free Cellular Imaging and Photodynamic Cancer Cell Ablation. Chem. Sci. 2018, 9, 3685–3693.
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88.
- Nolting, D.D.; Gore, J.C.; Pham, W. Near-Infrared Dyes: Probe Development and Applications in Optical Molecular Imaging. Curr. Org. Synth. 2011, 8, 521–534.
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2014, 43, 16–29.
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-Red to near Infrared Analyte-Responsive Fluorescent Probes Based on Organic Fluorophore Platforms for Fluorescence Imaging. Chem. Soc. Rev. 2013, 42, 622–661.
- Patel, N.; Pera, P.; Joshi, P.; Dukh, M.; Tabaczynski, W.A.; Siters, K.E.; Kryman, M.; Cheruku, R.R.; Durrani, F.; Missert, J.R.; et al. Highly Effective Dual-Function Near-Infrared (NIR) Photosensitizer for Fluorescence Imaging and Photodynamic Therapy (PDT) of Cancer. J. Med. Chem. 2016, 59, 9774–9787.
- Zhang, X.; Zhang, X.; Yang, B.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Fabrication of Aggregation Induced Emission Dye-Based Fluorescent Organic Nanoparticles via Emulsion Polymerization and Their Cell Imaging Applications. Polym. Chem. 2014, 5, 399–404.
- Zheng, Y.; Lu, H.; Jiang, Z.; Guan, Y.; Zou, J.; Wang, X.; Cheng, R.; Gao, H. Low-Power White Light Triggered AIE Polymer Nanoparticles with High ROS Quantum Yield for Mitochondria-Targeted and Image-Guided Photodynamic Therapy. J. Mater. Chem. B 2017, 5, 6277–6281.
- Yuan, Y.; Zhang, C.-J.; Kwok, R.T.K.; Xu, S.; Zhang, R.; Wu, J.; Tang, B.Z.; Liu, B. Light-Up Probe for Targeted and Activatable Photodynamic Therapy with Real-Time In Situ Reporting of Sensitizer Activation and Therapeutic Responses. Adv. Funct. Mater. 2015, 25, 6586–6595.
- Wang, H.; Liu, G.; Dong, S.; Xiong, J.; Du, Z.; Cheng, X. A PH-Responsive AIE Nanoprobe as a Drug Delivery System for Bioimaging and Cancer Therapy. J. Mater. Chem. B 2015, 3, 7401–7407.
- Wang, Z.; Yong, T.-Y.; Wan, J.; Li, Z.-H.; Zhao, H.; Zhao, Y.; Gan, L.; Yang, X.-L.; Xu, H.-B.; Zhang, C. Temperature-Sensitive Fluorescent Organic Nanoparticles with Aggregation-Induced Emission for Long-Term Cellular Tracing. ACS Appl. Mater. Interfaces 2015, 7, 3420–3425.
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592.
- Fox, R.G.; Lytle, N.K.; Jaquish, D.V.; Park, F.D.; Ito, T.; Bajaj, J.; Koechlein, C.S.; Zimdahl, B.; Yano, M.; Kopp, J.L.; et al. Image-Based Detection and Targeting of Therapy Resistance in Pancreatic Adenocarcinoma. Nature 2016, 534, 407–411.
- Jiang, Y.; Pu, K. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849.
- Urade, M.; Shinbo, T. Barium Appendicitis 1 Month After a Barium Meal. Int. Surg. 2013, 97, 296–298.
- Kurer, M.A.; Chintapatla, S. Intestinal Obstruction Due to Inspissated Barium. N. Engl. J. Med. 2007, 356, 1656.
- Cumberland, D.C. Optimum Viscosity of Barium Suspension for Use in the Double Contrast Barium Meal. Gastrointest. Radiol. 1977, 2, 169–174.
- Stringer, D.A.; Hassall, E.; Ferguson, A.C.; Cairns, R.; Nadel, H.; Sargent, M. Hypersensitivity Reaction to Single Contrast Barium Meal Studies in Children. Pediatr. Radiol. 1993, 23, 587–588.
- Nijhawan, S.; Kumpawat, S.; Mallikarjun, P.; Bansal, R.; Singla, D.; Ashdhir, P.; Mathur, A.; Rai, R.R. Barium Meal Follow through with Pneumocolon: Screening Test for Chronic Bowel Pain. World J. Gastroenterol. 2008, 14, 6694.
- Tada, S.; Iida, M.; Fuchigami, T.; Matsui, T.; Iwashita, A.; Yao, T.; Fujishima, M. Barium Meal Study for Amyloidosis of the Small Intestine: Measurements on Radiograph. Gastrointest. Radiol. 1990, 15, 320–324.
- Tai, C.-J.; Wang, W.; Huang, Y.-M. Barium Meal Peritonitis, Fatal Outcome in Unsuspected Small Bowel Perforation. Indian J. Surg. 2021, 83, 1589–1590.
- Elman, R.; MacLeod, J.W. Studies on the Neutralization of Gastric Acidity: Ewald Test Meal and X-Ray (Barium Meal) Studies in Patients with Duodenal Ulcer, Gastro-Jejunostomy and Gastric Resection. Am. J. Dig. Dis. Nutr. 1935, 2, 21–26.
- Zhang, Y.; West, J.J.; Mathur, R.; Xing, J.; Hogrefe, C.; Roselle, S.J.; Bash, J.O.; Pleim, J.E.; Gan, C.-M.; Wong, D.C. Long-term trends in the ambient PM2.5- and O3-related mortality burdens in the United States under emission reductions from 1990 to 2010. Atmos. Chem. Phys. 2018, 18, 15003–15016.
- Li, X.; Jin, L.; Kan, H. Air pollution: A global problem needs local fixes. Nature 2019, 570, 437–439.
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent Reconstitution on Deposition of PM2.5 in Lung and Extrapulmonary Organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493.




