
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barbara Demmig-Adams | -- | 2976 | 2023-05-25 05:12:14 | | | |
| 2 | Jason Zhu | Meta information modification | 2976 | 2023-05-26 04:05:50 | | |
Video Upload Options
Land plants adjust growth rate, protein content, and antioxidant content in response to their environment. These acclimatory adjustments in plant form and function may require several days and development of a new leaf. An apparent lesser need for such acclimation is demonstrated here for the floating aquatic plant Lemna minor with a focus on its response to the growth light environment. Relevant features of L. minor include unusually high growth rates, photosynthetic capacities, and protein content coupled with the ability to produce high levels of photoprotective xanthophylls (which are essential human micronutrients) across a wide range of growth light environments without compromising photosynthetic efficiency.
1. Capacity of Photosynthesis and Photosynthesis-Related Processes across Growth Light Intensity in Floating Aquatic Plants versus Land Plants
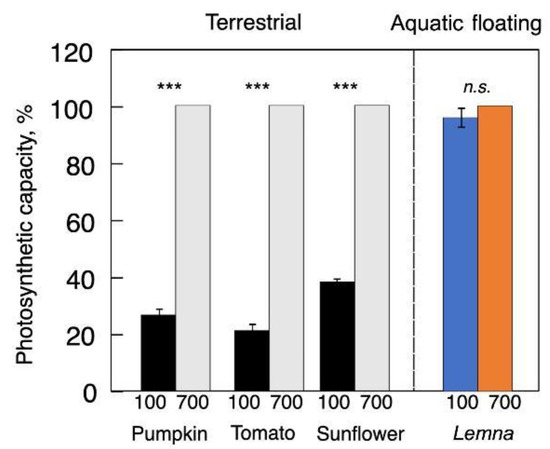
1.1. Photosynthetic Capacity
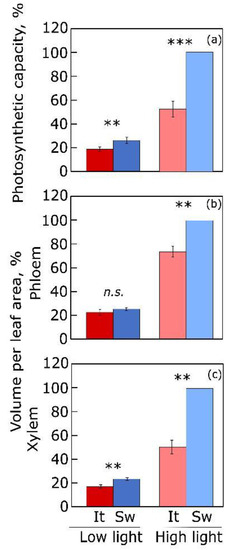
1.2. Vascular Infrastructure for Sugar and Water Transport in Land Plants
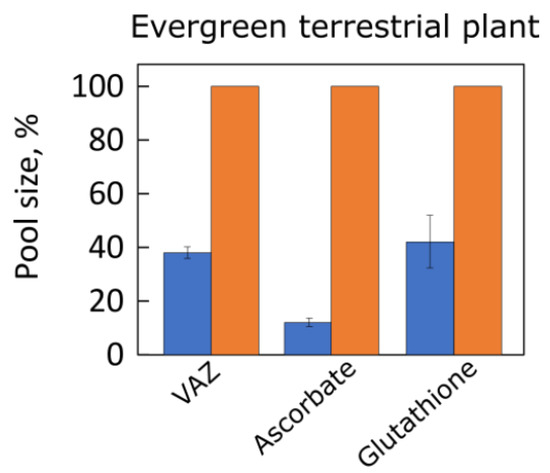
1.3. Antioxidants in Land Plants
2. Comparison of How Quickly Substantial Zeaxanthin Is Formed upon Sudden Transfer from Low to High Light in Land Plants versus Floating Aquatic Plants
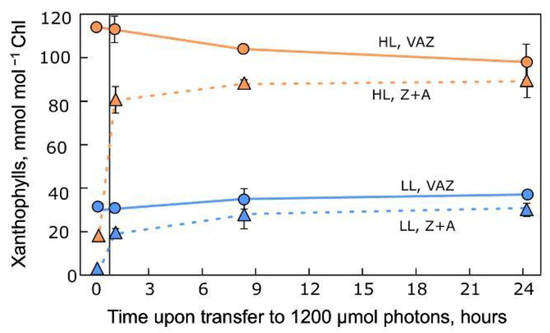
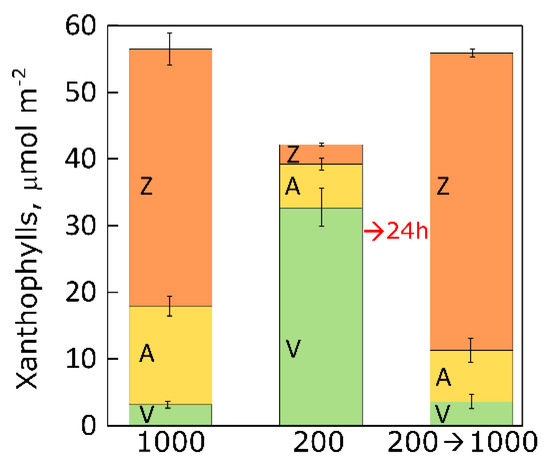
2.1. Changes in Xanthophyll Cycle Pool Size and Conversion in Terrestrial Plants upon Transfer
2.2. Changes in Xanthophyll Cycle Pool and Conversion in Lemna upon Transfer
3. Acclimation to Other Environmental Factors in Land Plants and Lemnaceae
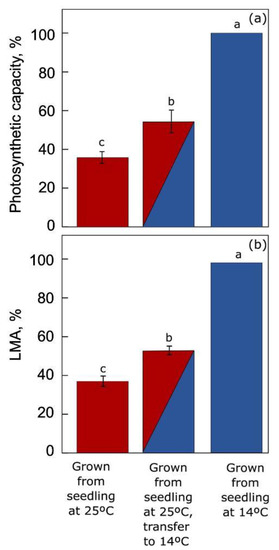
3.1. Temperature Acclimation in Land Plants
3.2. Response to Low or High Nitrogen Supply in Lemnaceae
3.3. Maintenance of Light-Harvesting Capacity in Lemnaceae despite Growth Arrest
References
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W., III. Environmental regulation of intrinsic photosynthetic capacity: An integrated view. Curr. Opin. Plant Biol. 2017, 37, 34–41.
- Nieuwland, M.; Geerdink, P.; Engelen-Smit, N.P.E.; van der Meer, I.M.; America, A.H.P.; Mes, J.J.; Kootstra, A.M.J.; Henket, J.T.M.M.; Mulder, W.J. Isolation and gelling properties of duckweed protein concentrate. ACS Food Sci. Technol. 2021, 1, 908–916.
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W. III. Growth and nutritional quality of Lemnaceae viewed comparatively in an ecological and evolutionary context. Plants 2022, 11, 145.
- Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering space with crops that produce ample oxygen and antioxidants. Oxygen 2022, 2, 211–226.
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the duckweed lemna that support rapid growth under extremes of light intensity. Cells 2021, 10, 1481.
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 1–14.
- Martindale, W.; Bowes, G. The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J. Exp. Bot. 1996, 47, 781–784.
- Oguchi, R.; Hikosaka, K.; Hirose, T. Leaf anatomy as a constraint for photosynthetic acclimation: Differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 2005, 28, 916–927.
- Oguchi, R.; Hikosaka, K.; Hirose, T. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ. 2003, 26, 505–512.
- Sims, D.A.; Pearcy, R.W. Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am. J. Bot. 1992, 79, 449–455.
- Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Demmig-Adams, B. Photosynthesis and foliar vascular adjustments to growth light intensity in summer annual species with symplastic and apoplastic phloem loading. J. Plant Physiol. 2021, 267, 153532.
- Adams, W.W., III; Stewart, J.J.; Polutchko, S.K.; Demmig-Adams, B. Leaf vasculature and the upper limit of photosynthesis. In The Leaf: A Platform for Performing Photosynthesis in Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 27–54.
- Cohu, C.M.; Muller, O.; Stewart, J.J.; Demmig-Adams, B.; Adams, W.W., III. Association between minor loading vein architecture and light- and CO2-saturated rates of photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front. Plant Sci. 2013, 22, 264.
- Stewart, J.J.; Polutchko, S.K.; Adams, W.W., III; Demmig-Adams, B. Acclimation of Swedish and Italian ecotypes of Arabidopsis thaliana to light intensity. Photosynth. Res. 2017, 134, 215–229.
- Adams, W.W., III; Stewart, J.J.; Cohu, C.M.; Muller, O.; Demmig-Adams, B. Habitat temperature and precipitation of Arabidopsis thaliana ecotypes determine the response of foliar vasculature, photosynthesis, and transpiration to growth temperature. Front. Plant Sci. 2016, 7, 1026.
- Landolt, E. Biosystematic investigations in the family of duckweeds (Lemnaceae), II: The family of Lemnaceae: A monographic study 1. Veröffentlichungen des Geobotanischen Institutes der ETH 1986, 95, 638.
- Sree, K.S.; Sudakaran, S.; Appenroth, K.J. How fast can angiosperms grow? Species and clonal diversity of growth rates in the genus Wolffia (Lemnaceae). Acta Physiol. Plant. 2015, 37, 204.
- Cedergreen, N.; Madsen, T.V. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol. 2002, 155, 285–292.
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a model plant system in the genomics and postgenomics era. Plant Cell. 2021, 33, 3207–3234.
- Kim, I.S. Development of the root system in Spirodela polyrhiza (L.) Schleiden (Lemnaceae). J. Plant Biol. 2007, 50, 540–547.
- Mateo-Elizalde, C.; Lynn, J.; Ernst, E.; Martienssen, R. Duckweeds. Curr. Biol. 2023, 33, 89–91.
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640.
- Müller-Moulé, P.; Havaux, M.; Niyogi, K.K. Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 2003, 133, 748–760.
- Neubauer, C.; Yamamoto, H.Y. Membrane barriers and Mehler-peroxidase reaction limit the ascorbate available for violaxanthin de-epoxidase activity in intact chloroplasts. Photosynth. Res. 1994, 39, 137–147.
- Müller-Schüssele, S.J.; Wang, R.; Gütle, D.D.; Romer, J.; Rodriguez-Franco, M.; Scholz, M.; Buchert, F.; Lüth, V.M.; Kopriva, S.; Dörmann, P.; et al. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. Plant J. 2020, 103, 1140–1154.
- Demmig-Adams, B.; Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III. History of excess-light exposure modulates extent and kinetics of fast-acting non-photochemical energy dissipation. Plant Physiol. Rep. 2022, 27, 560–572.
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273.
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An overview of nutritional and anti nutritional factors in green leafy vegetables. Hortic. Int. J. 2017, 1, 58–65.
- Diotallevi, C.; Angeli, A.; Vrhovsek, U.; Gobbetti, M.; Shai, I.; Lapidot, M.; Tuohy, K. Measuring phenolic compounds in Mankai: A novel polyphenol and amino rich plant protein source. Proc. Nutr. Soc. 2020, 79, E434.
- Yaskolka Meir, A.; Tuohy, K.; von Bergen, M.; Krajmalnik-Brown, R.; Heinig, U.; Zelicha, H.; Tsaban, G.; Rinott, E.; Kaplan, A.; Aharoni, A.; et al. The metabolomic-gut-clinical axis of mankai plant-derived dietary polyphenols. Nutrients 2021, 13, 1866.
- Hu, Z.; Fang, Y.; Yi, Z.; Tian, X.; Li, J.; Jin, Y.; He, K.; Liu, P.; Du, A.; Huang, Y.; et al. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT 2022, 153, 112477.
- Pagliuso, D.; Palacios Jara, C.E.; Grandis, A.; Lam, E.; Pena Ferreira, M.J.; Buckeridge, M.S. Flavonoids from duckweeds: Potential applications in the human diet. RSC Adv. 2020, 10, 44981–44988.
- Demmig-Adams, B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol. 1998, 39, 474–482.
- Demmig-Adams, B.; Winter, K.; Winkelmann, E.; Krüger, A.; Czygan, F.C. Photosynthetic characteristics and the ratios of chlorophyll, β-carotene, and the components of the xanthophyll cycle upon a sudden increase in growth light regime in several plant species. Bot. Acta 1989, 102, 319–325.
- Demmig-Adams, B.; Moeller, D.L.; Logan, B.A.; Adams, W.W., III. Positive correlation between levels of retained zeaxanthin + antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola (Hayata) Merrill. Planta 1998, 205, 367–374.
- Vialet-Chabrand, S.R.M.; Matthews, J.S.A.; McAusland, L.; Blatt, M.R.; Griffiths, H.; Lawson, T. Temporal dynamics of stomatal behavior: Modeling and implications for photosynthesis and water use. Plant Physiol. 2017, 174, 603–613.
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W., III. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88.
- Brodribb, T.J.; McAdam, S.A.M.; Carins Murphy, M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880.
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic modulation in response to plant activity and environment. In The Leaf: A Platform for Performing Photosynthesis; Adams, W.W., III, Terashima, I., Eds.; Advances in Photosynthesis and Respiration; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563.
- Cohu, C.M.; Lombardi, E.; Adams, W.W., III; Demmig-Adams, B. Increased nutritional quality of plants for long-duration spaceflight missions through choice of plant variety and manipulation of growth conditions. Acta Astronaut. 2014, 94, 799–806.
- Stewart, J.J.; Demmig-Adams, B.; Cohu, C.M.; Wenzl, C.A.; Muller, O.; Adams, W.W., III. Growth temperature impact on leaf form and function in Arabidopsis thaliana ecotypes from northern and southern Europe. Plant Cell Environ. 2016, 39, 1549–1558.
- Duong, T.P.; Tiedje, J.M. Nitrogen fixation by naturally occurring duckweed–cyanobacterial associations. Can. J. Microbiol. 1985, 31, 327–330.
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204.
- Polutchko, S.K.; Stewart, J.J.; Mcnamara, M.; Garcia, N.D.; López-Pozo, M.; Adams, W.W., III; Demmig-Adams, B. Lemna as a sustainable, highly nutritious crop: Nutrient production in different light environments. Nutraceuticals 2022, 2, 350–364.
- Roubeau Dumont, E.; Larue, C.; Pujol, B.; Lamaze, T.; Elger, A. Environmental variations mediate duckweed (Lemna minor L.) sensitivity to copper exposure through phenotypic plasticity. Environ. Sci. Pollut. Res. 2019, 26, 14106–14115.
- Logan, B.A.; Demmig-Adams, B.; Rosenstiel, T.N.; Adams, W.W., III. Effect of nitrogen limitation on foliar antioxidants in relationship to other metabolic characteristics. Planta 1999, 209, 213–220.
- Harper, J.E. Soil and symbiotic nitrogen requirements for optimum soybean production. Crop. Sci. 1974, 14, 255–260.
- Driever, S.M.; Van Nes, E.H.; Roijackers, R.M.M. Growth limitation of Lemna minor due to high plant density. Aquat. Bot. 2005, 81, 245–251.




