
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matteo Pellegrini | -- | 1823 | 2023-05-24 20:13:27 | | | |
| 2 | Sirius Huang | -10 word(s) | 1813 | 2023-05-26 09:25:45 | | |
Video Upload Options
Meshes, especially titanium ones, are being widely applied in oral surgery. In guided bone regeneration (GBR) procedures, their use is often paired with membranes, being resorbable or non-resorbable. However, they present some limitations, such as difficulty in the treatment of severe bone defects, alongside frequent mesh exposure. Customized meshes, produced by a full-digital process, have been recently introduced in GBR procedures. The main findings in recent years of clinical trials regarding patient-specific mesh produced by CAD/CAM and 3D printing workflow, made in titanium or even PEEK, applied to GBR surgeries, are described. The purpose is to analyze their clinical management, advantages, and complications.
1. Introduction
2. Bone Defect Dimension
3. Aesthetic Aspects
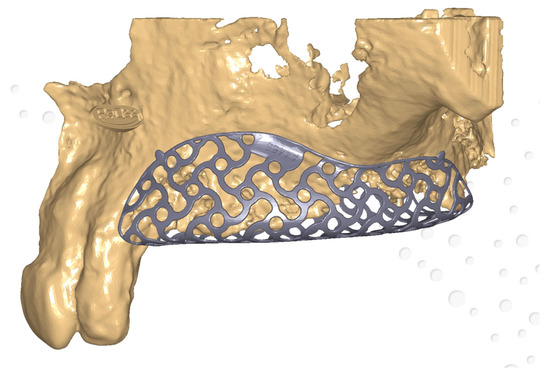
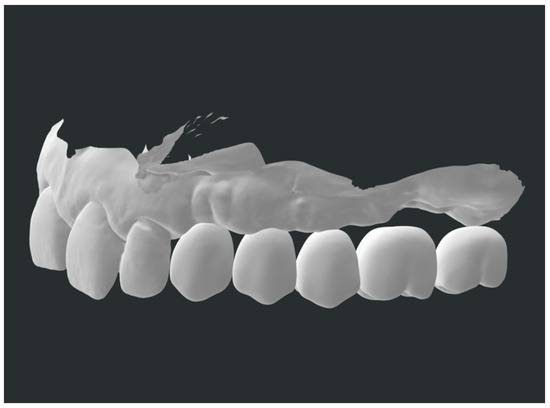
4. Biological Considerations
5. Clinical Success and Complications
6. Early and Late Complication Management
7. New Materials
References
- Hansson, S.; Halldin, A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J. Dent. Biomech. 2012, 3, 1758736012456543.
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23, 1–21.
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337.
- Bornstein, M.M.; Halbritter, S.; Harnisch, H.; Weber, H.P.; Buser, D. A retrospective analysis of patients referred for implant placement to a specialty clinic: Indications, surgical procedures, and early failures. Int. J. Oral Maxillofac. Implant. 2008, 23, 1109–1116.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576.
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. Biomed. Res. Int. 2015, 2015, 415289.
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.L.; Galindo-Moreno, P. Clinical Application of Mesenchymal Stem Cells and Novel Supportive Therapies for Oral Bone Regeneration. Biomed. Res. Int. 2015, 2015, 341327.
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. Latest Findings of the Regenerative Materials Application in Periodontal and Peri-Implant Surgery: A Scoping Review. Bioengineering 2022, 9, 594.
- Kormas, I.; Pedercini, A.; Alassy, H.; Wolff, L.F. The Use of Biocompatible Membranes in Oral Surgery: The Past, Present & Future Directions. A Narrative Review. Membranes 2022, 12, 841.
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56.
- Jung, G.; Jeon, J.; Hwang, K.; Park, C. Preliminary evaluation of a three-dimensional, customized, and preformed titanium mesh in peri-implant alveolar bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181–187.
- Badhey, A.; Kadakia, S.; Mourad, M.; Inman, J.; Ducic, Y. Calvarial Reconstruction. Semin. Plast. Surg. 2017, 31, 222–226.
- Konstantinidis, I.; Kumar, T.; Kher, U.; Stanitsas, P.D.; Hinrichs, J.E.; Kotsakis, G.A. Clinical results of implant placement in resorbed ridges using simultaneous guided bone regeneration: A multicenter case series. Clin. Oral Investig. 2015, 19, 553–559.
- Poli, P.P.; Beretta, M.; Cicciu, M.; Maiorana, C. Alveolar ridge augmentation with titanium mesh. A retrospective clinical study. Open Dent. J. 2014, 8, 148–158.
- Zhou, M.; Li, S.Y.; Terheyden, H.; Cao, S.S.; Che, Y.J.; Geng, Y.M. Particulate Coral Hydroxyapatite Sheltered by Titanium Mesh for Localized Alveolar Rehabilitation After Onlay Graft Failure: A Case Report. J. Oral Implantol. 2018, 44, 147–152.
- Maiorana, C.; Manfredini, M.; Beretta, M.; Signorino, F.; Bovio, A.; Poli, P.P. Clinical and Radiographic Evaluation of Simultaneous Alveolar Ridge Augmentation by Means of Preformed Titanium Meshes at Dehiscence-Type Peri-Implant Defects: A Prospective Pilot Study. Materials 2020, 13, 2389.
- Louis, P.J.; Gutta, R.; Said-Al-Naief, N.; Bartolucci, A.A. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J. Oral Maxillofac. Surg. 2008, 66, 235–245.
- Corinaldesi, G.; Pieri, F.; Sapigni, L.; Marchetti, C. Evaluation of survival and success rates of dental implants placed at the time of or after alveolar ridge augmentation with an autogenous mandibular bone graft and titanium mesh: A 3- to 8-year retrospective study. Int. J. Oral Maxillofac. Implant. 2009, 24, 1119–1128.
- Maiorana, C.; Santoro, F.; Rabagliati, M.; Salina, S. Evaluation of the use of iliac cancellous bone and anorganic bovine bone in the reconstruction of the atrophic maxilla with titanium mesh: A clinical and histologic investigation. Int. J. Oral Maxillofac. Implant. 2001, 16, 427–432.
- Roccuzzo, M.; Ramieri, G.; Bunino, M.; Berrone, S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: A controlled clinical trial. Clin. Oral Implant. Res. 2007, 18, 286–294.
- Poli, P.P.; Beretta, M.; Maiorana, C.; Souza, F.Á.; Bovio, A.; Manfredini, M. Therapeutic Strategies in the Management of Nonresorbable Membrane and Titanium Mesh Exposures Following Alveolar Bone Augmentation: A Systematic Scoping Review. Int. J. Oral Maxillofac. Implant. 2022, 37, 250–269.
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40.
- Okubo, T.; Tsukimura, N.; Taniyama, T.; Ishijima, M.; Nakhaei, K.; Rezaei, N.M.; Hirota, M.; Park, W.; Akita, D.; Tateno, A.; et al. Ultraviolet treatment restores bioactivity of titanium mesh plate degraded by contact with medical gloves. J. Oral Sci. 2018, 60, 567–573.
- Hartmann, A.; Hildebrandt, H.; Schmohl, J.U.; Kämmerer, P.W. Evaluation of risk parameters in bone regeneration using a customized titanium mesh: Results of a clinical study. Implant. Dent. 2019, 28, 543–550.
- Al-Ardah, A.J.; Alqahtani, N.; AlHelal, A.; Goodacre, B.J.; Swamidass, R.; Garbacea, A.; Lozada, J. Using Virtual Ridge Augmentation and 3-Dimensional Printing to Fabricate a Titanium Mesh Positioning Device: A Novel Technique Letter. J. Oral Implantol. 2018, 44, 293–299.
- Hartmann, A.; Seiler, M. Minimizing risk of customized titanium mesh exposures—A retrospective analysis. BMC Oral Health 2020, 20, 36.
- Inoue, K.; Nakajima, Y.; Omori, M.; Suwa, Y.; Kato-Kogoe, N.; Yamamoto, K.; Kitagaki, H.; Mori, S.; Nakano, H.; Ueno, T. Reconstruction of the Alveolar Bone Using Bone Augmentation With Selective Laser Melting Titanium Mesh Sheet: A Report of 2 Cases. Implant. Dent. 2018, 27, 602–607.
- Ciocca, L.; Fantini, M.; De Crescenzio, F.; Corinaldesi, G.; Scotti, R. Direct metal laser sintering (DMLS) of a customized titanium mesh for prosthetically guided bone regeneration of atrophic maxillary arches. Med. Biol. Eng. Comput. 2011, 49, 1347–1352.
- Kadkhodazadeh, M.; Amid, R.; Moscowchi, A. Management of extensive peri-implant defects with titanium meshes. Oral Maxillofac. Surg. 2021, 25, 561–568.
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin. Oral Investig. 2021, 25, 2969–2980.
- Yang, W.; Chen, D.; Wang, C.; Apicella, D.; Apicella, A.; Huang, Y.; Li, L.; Zheng, L.; Ji, P.; Wang, L.; et al. The effect of bone defect size on the 3D accuracy of alveolar bone augmentation performed with additively manufactured patient-specific titanium mesh. BMC Oral Health 2022, 22, 557.
- Boogaard, M.J.; Santoro, F.; Romanos, G.E. Mesh Ridge Augmentation Using CAD/CAM Technology for Design and Printing: Two Case Reports. Compend. Contin. Educ. Dent. 2022, 43, 654–663.
- Cucchi, A.; Bettini, S.; Corinaldesi, G. A novel technique for digitalisation and customisation of reinforced polytetrafluoroethylene meshes: Preliminary results of a clinical trial. Int. J. Oral Implantol. 2022, 15, 129–146.
- Lizio, G.; Pellegrino, G.; Corinaldesi, G.; Ferri, A.; Marchetti, C.; Felice, P. Guided bone regeneration using titanium mesh to augment 3-dimensional alveolar defects prior to implant placement. A pilot study. Clin. Oral Implant. Res. 2022, 33, 607–621.
- Chiapasco, M.; Casentini, P.; Tommasato, G.; Dellavia, C.; Del Fabbro, M. Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clin. Oral Implant. Res. 2021, 32, 498–510.
- Cuellar, C.N.; Gil, M.C.; Delgado, J.P.; Martínez, B.G.; Sanz, J.A.; de Atalaya, J.L.; Ochandiano, S.; Vila, C.N. Reconstrucción oromandibular con colgajo libre de peroné e implantes osteointegrados. Acta Otorrinolaringol. Esp. 2003, 54, 54–64.
- Navarro Cuéllar, C.; Tousidonis Rial, M.; Antúnez-Conde, R.; Ochandiano Caicoya, S.; Navarro Cuéllar, I.; Arenas de Frutos, G.; Sada Urmeneta, Á.; García-Hidalgo Alonso, M.I.; Navarro Vila, C.; Salmerón Escobar, J.I. Virtual Surgical Planning, Stereolitographic Models and CAD/CAM Titanium Mesh for Three-Dimensional Reconstruction of Fibula Flap with Iliac Crest Graft and Dental Implants. J. Clin. Med. 2021, 10, 1922.
- Iino, M.; Fukuda, M.; Nagai, H.; Hamada, Y.; Yamada, H.; Nakaoka, K.; Mori, Y.; Chikazu, D.; Saijo, H.; Seto, I.; et al. Evaluation of 15 mandibular reconstructions with Dumbach titan mesh-system and particulate cancellous bone and marrow harvested from bilateral posterior ilia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e1–e8.
- Gelețu, G.L.; Burlacu, A.; Murariu, A.; Andrian, S.; Golovcencu, L.; Baciu, E.R.; Maftei, G.; Onica, N. Customized 3D-Printed Titanium Mesh Developed for an Aesthetic Zone to Regenerate a Complex Bone Defect Resulting after a Deficient Odontectomy: A Case Report. Medicina 2022, 58, 1192.
- Nickenig, H.J.; Riekert, M.; Zirk, M.; Lentzen, M.P.; Zöller, J.E.; Kreppel, M. 3D-based buccal augmentation for ideal prosthetic implant alignment-an optimized method and report on 7 cases with pronounced buccal concavities. Clin. Oral Investig. 2022, 26, 3999–4010.
- Ghanaati, S.; Al-Maawi, S.; Conrad, T.; Lorenz, J.; Rössler, R.; Sader, R. Biomaterial-based bone regeneration and soft tissue management of the individualized 3D-titanium mesh: An alternative concept to autologous transplantation and flap mobilization. J. Craniomaxillofac. Surg. 2019, 47, 1633–1644.
- Tallarico, M.; Park, C.J.; Lumbau, A.I.; Annucci, M.; Baldoni, E.; Koshovari, A.; Meloni, S.M. Customized 3D-Printed Titanium Mesh Developed to Regenerate a Complex Bone Defect in the Aesthetic Zone: A Case Report Approached with a Fully Digital Workflow. Materials 2020, 13, 3874.
- Farronato, D.; Pasini, P.M.; Orsina, A.A.; Manfredini, M.; Azzi, L.; Farronato, M. Correlation between Buccal Bone Thickness at Implant Placement in Healed Sites and Buccal Soft Tissue Maturation Pattern: A Prospective Three-Year Study. Materials 2020, 13, 511.
- Chiapasco, M.; Casentini, P. Horizontal bone-augmentation procedures in implant dentistry: Prosthetically guided regeneration. Periodontol. 2000 2018, 77, 213–240.
- Dellavia, C.; Canciani, E.; Pellegrini, G.; Tommasato, G.; Graziano, D.; Chiapasco, M. Histological assessment of mandibular bone tissue after guided bone regeneration with customized computer-aided design/computer-assisted manufacture titanium mesh in humans: A cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 600–611.
- De Santis, D.; Luciano, U.; Donadello, D.; Faccioni, P.; Zarantonello, M.; Alberti, C.; Verlato, G.; Gelpi, F. Custom Bone Regeneration (CBR): An Alternative Method of Bone Augmentation-A Case Series Study. J. Clin. Med. 2022, 11, 4739.
- Lorenz, J.; Al-Maawi, S.; Sader, R.; Ghanaati, S. Individualized Titanium Mesh Combined With Platelet-Rich Fibrin and Deproteinized Bovine Bone: A New Approach for Challenging Augmentation. J. Oral Implantol. 2018, 44, 345–351.
- Ciocca, L.; Lizio, G.; Baldissara, P.; Sambuco, A.; Scotti, R.; Corinaldesi, G. Prosthetically CAD-CAM-Guided Bone Augmentation of Atrophic Jaws Using Customized Titanium Mesh: Preliminary Results of an Open Prospective Study. J. Oral Implantol. 2018, 44, 131–137.
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and horizontal ridge augmentation using customized CAD/CAM titanium mesh with versus without resorbable membranes. A randomized clinical trial. Clin. Oral Implant. Res. 2021, 32, 1411–1424.
- Lizio, G.; Corinaldesi, G.; Marchetti, C. Alveolar ridge reconstruction with titanium mesh: A three-dimensional evaluation of factors affecting bone augmentation. Int. J. Oral Maxillofac. Implant. 2014, 29, 1354–1363.
- Canullo, L.; Laino, L.; Longo, F.; Filetici, P.; D’Onofrio, I.; Troiano, G. Does chlorhexidine prevent complications in extractive, periodontal, and implant surgery? A systematic review and meta-analysis with trial sequential analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 1149–1158.
- Roccuzzo, M.; Ramieri, G.; Spada, M.C.; Bianchi, S.D.; Berrone, S. Vertical alveolar ridge augmentation by means of a titanium mesh and autogenous bone grafts. Clin. Oral Implant. Res. 2004, 15, 73–81.
- Al-Ardah, A.J.; AlHelal, A.; Proussaefs, P.; AlBader, B.; Al Humaidan, A.A.; Lozada, J. Managing titanium mesh exposure with partial removal of the exposed site: A case series study. J. Oral Implantol. 2017, 43, 482–490.
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810.
- Suresh, V.; Anolik, R.; Powers, D. The Utility of Polyether-Ether-Ketone Implants Adjacent to Sinus Cavities after Craniofacial Trauma. J. Oral Maxillofac. Surg. 2018, 76, 2361–2369.
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-Specific Surgical Implants Made of 3D Printed PEEK: Material, Technology, and Scope of Surgical Application. Biomed. Res. Int. 2018, 2018, 4520636.
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of three-dimensional bone augmentation of severely atrophied maxillary alveolar ridges using prebent titanium mesh vs customized poly-ether-ether-ketone (PEEK) mesh: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 960–967.
- Salem, D.; Reher, P.; Evans, J.L.; Mansour, M.H. Exploring digital technologies used in the design and manufacture of craniofacial implant surgical guides: A scoping review. J. Prosthet. Dent. 2023, in press.
- Thakur, J.; Parlani, S.; Shivakumar, S.; Jajoo, K. Accuracy of marginal fit of an implant-supported framework fabricated by 3D printing versus subtractive manufacturing technique: A systematic review and meta-analysis. J. Prosthet. Dent. 2023, 129, 301–309.
- Gallo, S.; Pascadopoli, M.; Pellegrini, M.; Pulicari, F.; Manfredini, M.; Zampetti, P.; Spadari, F.; Maiorana, C.; Scribante, A. CAD/CAM Abutments versus Stock Abutments: An Update Review. Prosthesis 2022, 4, 468–479.
- Cabello-Domínguez, G.; Pérez-López, J.; Veiga-López, B.; González, D.; Revilla-León, M. Maxillary zirconia and mandibular composite resin-lithium disilicate-modified PEEK fixed implant-supported restorations for a completely edentulous patient with an atrophic maxilla and mandible: A clinical report. J. Prosthet. Dent. 2020, 124, 403–410.




